Figure 2. Modified concurrent dual mode of KCNQ channel inhibition .
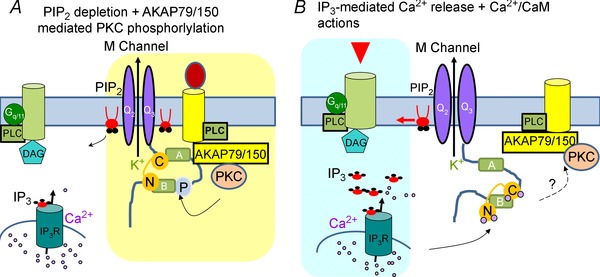
In this model, phosphorylation of KCNQ channels functions to reduce their PIP2 affinity, priming them to small changes in PIP2 abundance induced by physiological stimulation of receptors. A, activation of receptors that are part of AKAP79150–KCNQ complexes rapidly depletes PIP2 and activates AKAP79/150‐anchored PKC, which phosphorylates KCNQ subunits near the CaM binding site on the B helix, altering the configuration of CaM and KCNQ channels. KCNQ channels thus sense both PIP2 depletion and phosphorylation‐mediated PIP2‐affinity reduction. B, activation of receptors located close to IP3 receptors, but outside of the AKAP–KCNQ complexes, induces intracellular Ca2+ rises and Ca2+ binding to CaM, which undergoes a conformational change on the overlapping binding sites with AKAP150 on the carboxy‐terminus of KCNQ channels. This induces a lowered interaction between KCNQ and AKAP79/150, thus preventing PKC phosphorylation.
