The title complex is one of the few structurally characterized coordination compounds of an organotin(IV) trihalide with 2,2′-biypridine. Its distorted octahedral geometry shows a meridional arrangement of the I atoms and the methyl group is in-plane with the five-membered chelate ring. Directional intermolecular interactions are restricted to weak I⋯H van der Waals contacts.
Keywords: crystal structure; methyltin(IV) triiode; 2,2′-bipyridine; coordination compound
Abstract
The title compound, (2,2′-bipyridine-κ2 N,N′)triiodidomethyltin(IV), [Sn(CH3)I3(C10H8N2)], crystallizing in the non-centrosymmetric orthorhombic space group Pca21 as an inversion twin, represents one of the few structurally characterized coordination compounds of an organotin(IV) trihalide with 2,2′-biypridine. Its distorted octahedral geometry shows a meridional arrangement of the I atoms and the methyl group is in-plane with the five-membered chelate ring. Asymmetric bonding of the biypridine ligand to the tin(IV) atom is reflected by different Sn—N bond lengths [2.268 (4) Å versus 2.293 (4) Å] and caused by the static trans effect of the methyl group. Sn—I bond lengths show some differences with respect to their orientation to the methyl group or the bipyridine ligand, respectively. Angular distortions in the coordination sphere of the SnIV atom mainly arise from the large I atoms. Distortion of the 2,2′-bipyridine ligand as a result of its coordination to the SnIV atom are described by the twisting angle of 2.5 (2)° between the least-squares planes of the two pyridine rings, as well as by the angle of 6.2 (2)° between the two lines through the pyridine-connecting C atoms and the para-orientated C atoms. Directional intermolecular interactions are restricted to weak I⋯H van der Waals contacts.
Chemical context
Tin(IV) halides and organotin(IV) halides, R
4-nSnHaln with n = 1,2,3,4 and Hal = F, Cl, Br, I, show a graduated Lewis acid activity towards Lewis bases. As early as 1898, Werner and Pfeiffer stated that the acidity is decreased in the sequence: SnHal4 > RSnHal3 > R
2SnHal2 > R
3SnHal (Werner & Pfeiffer, 1898 ▸). Although monoorganotin(IV) halides show the highest Lewis acidity among the organotin(IV) halides, only a few complexes have been prepared and even fewer have been structurally characterized, in contrast to the situation in case of diorganotin(IV) dihalides. The few examples that have been structurally investigated are dominated by monodentate Lewis bases with O or N as coordination donors, whereas corresponding bidentate ligands are inadequately represented. Currently, there are only five coordination compounds of monoorganotin(IV) trihalides with bidentate N,N-chelating ligands listed in the Cambridge Crystallographic Database (Version 5.36, last update May 2015; Groom & Allen, 2014 ▸) but only three, BzlSnCl3(phen) (Hall & Tiekink, 1996 ▸), 1, EtSnI3(bipy) (Paseshnichenko et al., 1984 ▸), 2, R′SnCl3(bipy) with R′ = 3-(4-methoxybenzyl)cyclopentadienyl (Gleeson et al., 2008 ▸), 3, exhibit an almost planar backbone of the ligand as is characteristic for 2,2′-bipyridine (bipy) or 1,10-phenanthroline (phen). From a fundamental point of view, such complexes are of special interest, because of two possible steroisomers which differ in the position of the organic substituent in relation to the plane of the ligand (in-plane or perpendicular) while the three halide atoms adopt a meridional or facial orientation. The majority of all complexes investigated exhibit a meridional arrangement of the halide atoms, only 3 features a facial one.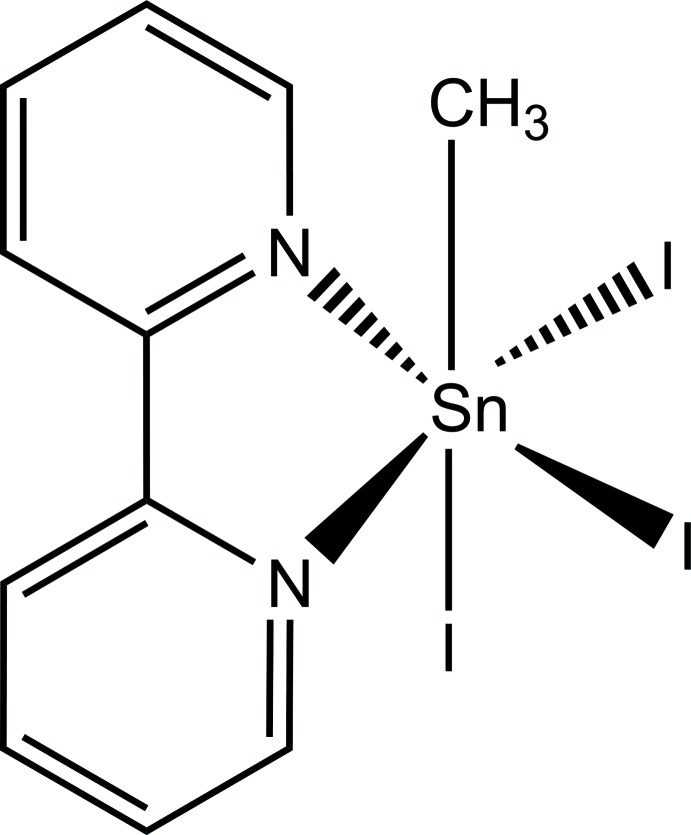
During a systematic study (Reuter et al., 2011 ▸) on the solid-state structures of diorganotin(IV) dihalides, R 2SnHal2, we were interested in methylphenyldiiodidotin(IV), MePhSnI2, because of the unique crystal structure of the corresponding dichloride (Amini et al., 1987 ▸). Experiments to achieve this diiodide from the corresponding oxide by reaction with aqueous ammonium iodide, however, failed as the resulting liquid turned out to be a mixture of two or more different unknown organotin species which could not purified by distillation. We therefore tried to synthesize derivates of these compounds by adding 2,2′-bipyridine to the mixtures in the hope of obtaining single crystals for identification. Indeed, the synthesis succeeded and we found two different kinds of single crystals, orange needles of the title compound and red blocks of the 2,2′-bipyridine complex of dimethyldiiodidotin(IV), Me2SnI2·bipy, the structure of which was also confirmed by X-ray diffraction (Reuter & Reichelt, unpublished results).
Structural commentary
The tin(IV) atom of the title compound is distorted octahedrally coordinated with the methyl group in plane with the chelating ligand and the iodine atoms in a meridional arrangement (Fig. 1 ▸). Although the formation of the five-membered chelate ring between the bidentate 2,2′-biypridine ligand and the tin(IV) atom provides the complex a certain rigidity, there remains enough conformational adaptability to react flexibly towards electronic as well as steric intramolecular or intermolecular demands. Since the pioneering work of Buslaev et al. (1989 ▸), the important role of electronic effects on bond lengths in complexes of monoorganontin(IV) halides in particular and monoorganotin(IV) compounds in general (Reuter & Ye, 2013 ▸; Reichelt & Reuter, 2013 ▸) is well established and introduced into the literature as the static trans-effect meaning that a bond trans to the organic group is shortened in comparison to a comparable bond in cis position. As a result, the 2,2′-bipyridine ligand of the title compound bonds asymmetrically to the tin(IV) atom: the Sn—N bond trans to the methyl group [d(Sn—N) = 2.268 (4) Å] is shorter than the other [d(Sn—N) = 2.293 (4) Å].
Figure 1.
Thermal ellipsoid model of the asymmetric unit in the crystal structure of the title compound with the atomic numbering scheme used. With exception of the H atoms, which are shown as spheres of arbitrary radius, all other atoms are drawn with displacement ellipsoids at the 50% probability level.
The lengths of the three Sn—I bonds are very similar, although there is a significant difference between the Sn—I bond trans to the bipyridine ligand [d(Sn1—I1) = 2.8041 (5) Å] and the two cis orientated Sn—I bonds [mean value: d(Sn—I) = 2.853 (7) Å]. Similar Sn—I bond lengths (2.808, 2.838–2.878 Å) are found in the ethyl compound 2. All in all, these Sn—I bonds of sixfold-coordinated tin(IV) are about 0.2 Å longer than for the tin(IV) atom in the tetrahedral environment of SnI4, where a mean value of 2.661 Å has been observed (Reuter & Pawlak, 2001 ▸).
In comparison with the Sn—C bond length of the corresponding dimethyldiiodidotin(IV) compound [2.122 (3) Å; Reuter & Reichelt, unpublished], that of the title compound is rather long [2.179 (5) Å]. In the corresponding ethyl compound 2, the bond is even longer (2.199 Å). Whether this reflects a general trend is difficult to decide, because no other precise structural data of complexes of monoorganotin(IV) triiodides are available.
Other distortions of the octahedral coordination around the central tin(IV) atom concern bond angles which deviate significantly from the bond angles in a regular octahedron. The distortions are caused mainly by the large iodine atoms, which demand the most space in the environment of the tin atom, with the result that the bond angles between the iodine atoms themselves, as well as the bond angles between the iodine atoms and the methyl group, are significantly larger than 90° [96.9 (1), 94.9 (2), 93.06 (1)°; Table 1 ▸]. As a consequence, the axis through the iodine atoms cis to the bipy ligand is bent [167.04 (2)°] in direction of the chelate ligand. All these bond-angle distortions, however, take place within the planes these four atoms are involved in [I1–Sn1–C11/I2–Sn1–I3] so that these planes are almost perpendicular to each other [dihedral angle: 89.44 (7)°] (Fig. 2 ▸). In contrast, the bipyridine ligand adopts an inclined orientation [dihedral angles: 86.32 (3)° with I2–Sn1–I3; 4.24 (8)° with I1–Sn1–C11].
Table 1. Selected geometric parameters (Å, °).
| Sn1—C11 | 2.179 (5) | Sn1—I1 | 2.8041 (5) |
| Sn1—N1 | 2.268 (4) | Sn1—I3 | 2.8476 (4) |
| Sn1—N2 | 2.293 (4) | Sn1—I2 | 2.8580 (4) |
| C11—Sn1—I1 | 96.85 (14) | I1—Sn1—I2 | 94.416 (13) |
| C11—Sn1—I3 | 94.87 (15) | I3—Sn1—I2 | 167.039 (17) |
| I1—Sn1—I3 | 93.064 (13) |
Figure 2.
Displacement ellipsoid model of the title compound looking down the direction of the planes I1–Sn1–C11 and I2–Sn1–I3 and their dihedral angle (all in blue); orientation of the least-squares plane through all atoms of the 2,2′-bipyridine ligand in relation to these planes with the corresponding dihedral angles (all in red).
The bipyridine ligand itself shows the typical bond lengths and angles: d(C—N) = 1.346, d(C—C)arom = 1.385, C—N—C = 119.3°, all mean values. As usual, the shorter C—N bonds and angles at nitrogen are compensated by larger bond angles at the carbon atoms so that planarity of both pyridine moieties is retained [deviations from least-squares plane (Å): N1 = −0.005 (3), C1 = −0.002 (3), C2 = 0.005 (4), C3 = −0.001 (4), C4 = −0.007 (3), C5 = 0.010 (3); N2 = −0.002 (3), C7 = 0.004 (3), C8 = −0.003 (3), C9 = 0.001 (3), C10 = 0.001 (3), C6 = −0.001 (3)]. The C—C single bond between the two pyridine groups has a length of 1.488 (6) Å. The interaction of the ligand with the tin(IV) atom, however, produces some distortions affecting the planarity of the ligand as a whole, as well as the orientation of the two pyridine rings in relation to each other. Twisting of the bipyridine ligand is best described by the dihedral angle of 2.5 (2)° (Fig. 3 ▸) between the least-squares planes of the pyridine rings, while its bending (Fig. 4 ▸) can be quantitatively described using the angle of 6.2 (2)° between the lines through the linking carbon atoms (C5 and C6) and their para-orientated counterparts (C2 and C8).
Figure 3.
Displacement ellipsoid model of the title compound looking onto the biypridine ligand, showing its twisting described by the dihedral angle between the least-squares planes through the two pyridine moieties of this ligand.
Figure 4.
Displacement ellipsoid model of the title compound looking onto the bipyridine ligand, showing its bending described by the angle between the two lines through the connecting and the para-oriented carbon atoms.
Supramolecular features
In the solid state, there are only weak interactions between the complexes. No π–π interactions between the aromatic rings or Sn⋯I interactions between neighboring molecules are observed (Fig. 5 ▸). I⋯H van der Waals type contacts are the only type of directional interactions between molecules. The shortest interaction [3.047 Å] is found between an H atom (H10) of the bipyridine ligand of one molecule with an iodine atom (I1) of the neighboring molecule almost colinear with the c axis (Fig. 5 ▸). Because of space-group symmetry, the strands of molecules connected this way are arranged in V-shaped pairs [opening angle about 52°] with an offset of c/2 between individual I⋯H connected strands. Along the b-axis direction, neighboring pairs of molecules are connected via somewhat longer I⋯H contacts [3.162 Å, I3⋯H2], while along the a-axis direction there are no contacts shorter than 3.2 Å.
Figure 5.
Perspective view of the crystal structure seen parallel to [001], looking down the strands resulting from short I⋯H van der Waals contacts between bipyridine and iodine of neighbouring complexes. All van der Waals contacts shorter 3.2 Å are drawn as dashed blue lines.
Synthesis and crystallization
In a typical experiment, a suspension of 4.1 g (18 mmol) MePhSnO and 5.6 g (50 mmol, excess) NH4I in toluene was heated to reflux of the solvent for 24 h using a Soxhlet extractor filled with silica gel for water adsorption. After evaporation of the organic solvent, the remaining liquid was proved by 13C NMR spectroscopy to be composed of at least two different organotin(IV) species. Attempts to separate these compounds by distillation failed. Re-dissolution of the residue in toluene, and addition of 2,2′-bipyridine followed by slow evaporation of the organic solvent, however, resulted in the formation of two different crystal forms; orange needles of the title compound and red blocks of Me2SnI2·bipy. Unfortunately, further attempts to separate larger amounts of the different species for further characterization were unsuccessful.
Refinement details
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The title compound crystallizes in the non-centrosymmetric, orthorhombic space group Pca21. As the Flack parameter deviates significantly from zero, the structure was refined as an inversion twin with a twin-factor of 0.12 (3). All hydrogen atoms could be localized in difference Fourier syntheses but were refined in geometric positions riding on the carbon atoms with C—H distances of 0.98 Å (–CH3) and 0.95 Å (–CHarom) and with U iso(H) = 1.2U eq(C). Reflection 2 0 0 was omitted because it was affected by the beam stop.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Sn(CH3)I3(C10H8N2)] |
| M r | 670.61 |
| Crystal system, space group | Orthorhombic, P c a21 |
| Temperature (K) | 100 |
| a, b, c (Å) | 23.5604 (4), 7.0367 (3), 9.5792 (4) |
| V (Å3) | 1588.11 (10) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 7.42 |
| Crystal size (mm) | 0.23 × 0.15 × 0.11 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2009 ▸) |
| T min, T max | 0.285, 0.501 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 48663, 4026, 3990 |
| R int | 0.039 |
| (sin θ/λ)max (Å−1) | 0.677 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.016, 0.037, 1.21 |
| No. of reflections | 4026 |
| No. of parameters | 156 |
| No. of restraints | 1 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.61, −0.81 |
| Absolute structure | Refined as an inversion twin. |
| Absolute structure parameter | 0.12 (3) |
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S2056989015022975/zl2654sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015022975/zl2654Isup2.hkl
CCDC reference: 1439787
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank the state of Lower-Saxony and the Deutsche Forschungsgemeinschaft for funding the diffractometer.
supplementary crystallographic information
Crystal data
| [Sn(CH3)I3(C10H8N2)] | Dx = 2.805 Mg m−3 |
| Mr = 670.61 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, Pca21 | Cell parameters from 9469 reflections |
| a = 23.5604 (4) Å | θ = 2.7–28.8° |
| b = 7.0367 (3) Å | µ = 7.42 mm−1 |
| c = 9.5792 (4) Å | T = 100 K |
| V = 1588.11 (10) Å3 | Block, orange |
| Z = 4 | 0.23 × 0.15 × 0.11 mm |
| F(000) = 1200 |
Data collection
| Bruker APEXII CCD diffractometer | 3990 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.039 |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | θmax = 28.8°, θmin = 2.7° |
| Tmin = 0.285, Tmax = 0.501 | h = −31→31 |
| 48663 measured reflections | k = −9→9 |
| 4026 independent reflections | l = −12→11 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.016 | H-atom parameters constrained |
| wR(F2) = 0.037 | w = 1/[σ2(Fo2) + (0.0111P)2 + 2.8899P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.21 | (Δ/σ)max < 0.001 |
| 4026 reflections | Δρmax = 0.61 e Å−3 |
| 156 parameters | Δρmin = −0.81 e Å−3 |
| 1 restraint | Absolute structure: Refined as an inversion twin. |
| Primary atom site location: structure-invariant direct methods | Absolute structure parameter: 0.12 (3) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refined as a 2-component inversion twin. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Sn1 | 0.36213 (2) | 0.17120 (4) | 0.23984 (4) | 0.01142 (6) | |
| C11 | 0.3258 (2) | −0.0290 (8) | 0.3886 (6) | 0.0208 (11) | |
| H11 | 0.3094 | −0.1370 | 0.3381 | 0.025* | |
| H12 | 0.2960 | 0.0344 | 0.4428 | 0.025* | |
| H13 | 0.3555 | −0.0744 | 0.4518 | 0.025* | |
| I1 | 0.38951 (2) | 0.45560 (5) | 0.43351 (4) | 0.01814 (7) | |
| I2 | 0.47258 (2) | 0.00336 (5) | 0.24365 (4) | 0.01947 (7) | |
| I3 | 0.25648 (2) | 0.34316 (4) | 0.16992 (3) | 0.01643 (7) | |
| N1 | 0.39831 (16) | 0.3374 (6) | 0.0575 (4) | 0.0115 (7) | |
| C1 | 0.4260 (2) | 0.5041 (7) | 0.0732 (5) | 0.0145 (9) | |
| H1 | 0.4291 | 0.5579 | 0.1639 | 0.017* | |
| C2 | 0.4501 (2) | 0.5987 (8) | −0.0381 (5) | 0.0174 (10) | |
| H2 | 0.4696 | 0.7152 | −0.0241 | 0.021* | |
| C3 | 0.4455 (2) | 0.5221 (8) | −0.1699 (6) | 0.0187 (10) | |
| H3 | 0.4615 | 0.5851 | −0.2482 | 0.022* | |
| C4 | 0.4168 (2) | 0.3504 (7) | −0.1865 (5) | 0.0154 (9) | |
| H4 | 0.4126 | 0.2957 | −0.2765 | 0.019* | |
| C5 | 0.39433 (18) | 0.2602 (6) | −0.0701 (6) | 0.0133 (8) | |
| C6 | 0.36488 (19) | 0.0737 (6) | −0.0814 (5) | 0.0126 (9) | |
| N2 | 0.34677 (16) | −0.0009 (6) | 0.0402 (4) | 0.0123 (8) | |
| C7 | 0.3195 (2) | −0.1687 (7) | 0.0394 (6) | 0.0167 (10) | |
| H7 | 0.3073 | −0.2213 | 0.1256 | 0.020* | |
| C8 | 0.3088 (2) | −0.2670 (7) | −0.0824 (6) | 0.0185 (10) | |
| H8 | 0.2890 | −0.3846 | −0.0803 | 0.022* | |
| C9 | 0.3276 (2) | −0.1905 (8) | −0.2087 (6) | 0.0194 (10) | |
| H9 | 0.3210 | −0.2559 | −0.2939 | 0.023* | |
| C10 | 0.3558 (2) | −0.0188 (7) | −0.2079 (6) | 0.0172 (10) | |
| H10 | 0.3688 | 0.0358 | −0.2927 | 0.021* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Sn1 | 0.01426 (13) | 0.01016 (13) | 0.00985 (14) | −0.00100 (10) | 0.00007 (12) | −0.00058 (13) |
| C11 | 0.024 (3) | 0.024 (3) | 0.014 (2) | −0.001 (2) | 0.003 (2) | −0.003 (2) |
| I1 | 0.02387 (15) | 0.01725 (13) | 0.01331 (14) | −0.00236 (13) | −0.00016 (12) | −0.00381 (14) |
| I2 | 0.01713 (13) | 0.01535 (13) | 0.02594 (16) | 0.00278 (10) | −0.00681 (14) | −0.00460 (14) |
| I3 | 0.01422 (13) | 0.01633 (14) | 0.01874 (15) | 0.00167 (11) | 0.00060 (12) | −0.00138 (13) |
| N1 | 0.0111 (16) | 0.0113 (18) | 0.0123 (19) | −0.0006 (14) | −0.0006 (15) | 0.0025 (15) |
| C1 | 0.015 (2) | 0.013 (2) | 0.015 (2) | −0.0037 (17) | 0.0007 (18) | −0.0007 (19) |
| C2 | 0.017 (2) | 0.015 (2) | 0.020 (3) | −0.0011 (17) | 0.0026 (19) | 0.002 (2) |
| C3 | 0.021 (2) | 0.020 (3) | 0.016 (2) | 0.0001 (19) | 0.0066 (19) | 0.007 (2) |
| C4 | 0.021 (2) | 0.016 (2) | 0.009 (2) | 0.0008 (19) | 0.0006 (18) | −0.0012 (18) |
| C5 | 0.0101 (18) | 0.013 (2) | 0.016 (2) | −0.0005 (15) | −0.0008 (18) | 0.003 (2) |
| C6 | 0.014 (2) | 0.013 (2) | 0.011 (2) | 0.0007 (15) | −0.0024 (17) | −0.001 (2) |
| N2 | 0.0126 (18) | 0.0106 (19) | 0.014 (2) | 0.0025 (14) | 0.0006 (16) | 0.0002 (16) |
| C7 | 0.017 (2) | 0.015 (2) | 0.018 (3) | −0.0001 (18) | −0.0003 (19) | 0.002 (2) |
| C8 | 0.019 (2) | 0.013 (2) | 0.023 (3) | −0.0009 (18) | −0.006 (2) | −0.004 (2) |
| C9 | 0.024 (2) | 0.016 (2) | 0.019 (2) | 0.0002 (19) | −0.006 (2) | −0.0059 (19) |
| C10 | 0.020 (2) | 0.016 (2) | 0.015 (2) | 0.0007 (19) | −0.0017 (18) | −0.0027 (19) |
Geometric parameters (Å, º)
| Sn1—C11 | 2.179 (5) | C3—C4 | 1.393 (7) |
| Sn1—N1 | 2.268 (4) | C3—H3 | 0.9500 |
| Sn1—N2 | 2.293 (4) | C4—C5 | 1.388 (7) |
| Sn1—I1 | 2.8041 (5) | C4—H4 | 0.9500 |
| Sn1—I3 | 2.8476 (4) | C5—C6 | 1.488 (6) |
| Sn1—I2 | 2.8580 (4) | C6—N2 | 1.347 (7) |
| C11—H11 | 0.9800 | C6—C10 | 1.392 (7) |
| C11—H12 | 0.9800 | N2—C7 | 1.344 (6) |
| C11—H13 | 0.9800 | C7—C8 | 1.379 (8) |
| N1—C5 | 1.341 (7) | C7—H7 | 0.9500 |
| N1—C1 | 1.351 (6) | C8—C9 | 1.396 (8) |
| C1—C2 | 1.379 (7) | C8—H8 | 0.9500 |
| C1—H1 | 0.9500 | C9—C10 | 1.379 (7) |
| C2—C3 | 1.377 (8) | C9—H9 | 0.9500 |
| C2—H2 | 0.9500 | C10—H10 | 0.9500 |
| C11—Sn1—N1 | 169.90 (18) | C1—C2—H2 | 120.4 |
| C11—Sn1—N2 | 98.16 (18) | C2—C3—C4 | 118.8 (5) |
| N1—Sn1—N2 | 71.89 (15) | C2—C3—H3 | 120.6 |
| C11—Sn1—I1 | 96.85 (14) | C4—C3—H3 | 120.6 |
| N1—Sn1—I1 | 93.16 (11) | C5—C4—C3 | 119.4 (5) |
| N2—Sn1—I1 | 164.86 (10) | C5—C4—H4 | 120.3 |
| C11—Sn1—I3 | 94.87 (15) | C3—C4—H4 | 120.3 |
| N1—Sn1—I3 | 85.89 (10) | N1—C5—C4 | 121.3 (4) |
| N2—Sn1—I3 | 83.69 (10) | N1—C5—C6 | 117.2 (5) |
| I1—Sn1—I3 | 93.064 (13) | C4—C5—C6 | 121.5 (5) |
| C11—Sn1—I2 | 94.73 (15) | N2—C6—C10 | 121.4 (4) |
| N1—Sn1—I2 | 83.15 (10) | N2—C6—C5 | 115.4 (4) |
| N2—Sn1—I2 | 86.34 (10) | C10—C6—C5 | 123.2 (5) |
| I1—Sn1—I2 | 94.416 (13) | C7—N2—C6 | 119.3 (4) |
| I3—Sn1—I2 | 167.039 (17) | C7—N2—Sn1 | 123.0 (3) |
| Sn1—C11—H11 | 109.5 | C6—N2—Sn1 | 117.7 (3) |
| Sn1—C11—H12 | 109.5 | N2—C7—C8 | 122.2 (5) |
| H11—C11—H12 | 109.5 | N2—C7—H7 | 118.9 |
| Sn1—C11—H13 | 109.5 | C8—C7—H7 | 118.9 |
| H11—C11—H13 | 109.5 | C7—C8—C9 | 118.8 (4) |
| H12—C11—H13 | 109.5 | C7—C8—H8 | 120.6 |
| C5—N1—C1 | 119.2 (4) | C9—C8—H8 | 120.6 |
| C5—N1—Sn1 | 117.8 (3) | C10—C9—C8 | 119.0 (5) |
| C1—N1—Sn1 | 122.9 (3) | C10—C9—H9 | 120.5 |
| N1—C1—C2 | 122.1 (5) | C8—C9—H9 | 120.5 |
| N1—C1—H1 | 118.9 | C9—C10—C6 | 119.3 (5) |
| C2—C1—H1 | 118.9 | C9—C10—H10 | 120.4 |
| C3—C2—C1 | 119.2 (5) | C6—C10—H10 | 120.4 |
| C3—C2—H2 | 120.4 |
References
- Amini, M. M., Holt, E. M. & Zuckerman, J. J. (1987). J. Organomet. Chem. 327, 147–155.
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bruker (2009). APEX2, SADABS and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Buslaev, Yu. A., Kravchenko, E. A., Burtzev, M. Yu. & Aslanov, L. A.(1989). Coord. Chem. Rev. 93, 185–204.
- Gleeson, B., Claffey, J., Ertler, D., Hogan, M., Müller-Bunz, H., Paradisi, F., Wallis, D. & Tacke, M. (2008). Polyhedron, 27, 3619–3624.
- Groom, C. R. & Allen, F. H. (2014). Angew. Chem. Int. Ed. 53, 662–671. [DOI] [PubMed]
- Hall, V. J. & Tiekink, E. R. T. (1996). Acta Cryst. C52, 2141–2143.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Paseshnichenko, K. A., Aslanov, L. A., Yatsenko, A. V. & Medvedev, S. V. (1984). Koord. Khim. 10, 1279–1284.
- Reichelt, M. & Reuter, H. (2013). Acta Cryst. E69, m4. [DOI] [PMC free article] [PubMed]
- Reuter, H. & Pawlak, R. (2001). Z. Anorg. Allg. Chem. 216, 34–38.
- Reuter, H. & Ye, F. (2013). Main Group Met. Chem. 36, 225–227.
- Reuter, H., Ye, F., Reichelt, M., Vages, J., Osthaar, S. & Schwitke, S. (2011). Acta Cryst. A67, C729–C730.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Werner, A. & Pfeiffer, P. (1898). Z. Anorg. Chem. 17, 82–110.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S2056989015022975/zl2654sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015022975/zl2654Isup2.hkl
CCDC reference: 1439787
Additional supporting information: crystallographic information; 3D view; checkCIF report







