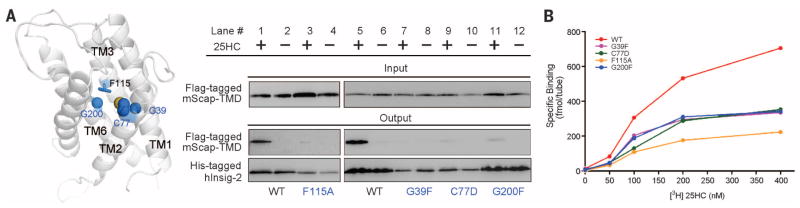
Fig. 4. Identification of residues involved in 25HC binding based on the homologous model of Insig-2.
(A) Identification of Insig-2 pocket residues that may contribute to 25HC binding. The pocket residues that are in the vicinity of Phe115 were analyzed. Single point mutations G39F, C77D, or G200F resulted in compromised mScap-TMD binding in the presence of 25HC. These three residues and Phe115 are positioned at a similar height within the central pocket of the structural model of Insig-2. (B) The measurement of direct binding between [3H]25HC and indicated Insig-2 variants. The experiments were performed following the identical protocol as reported previously (24). The data points represent the average of duplicate assays.