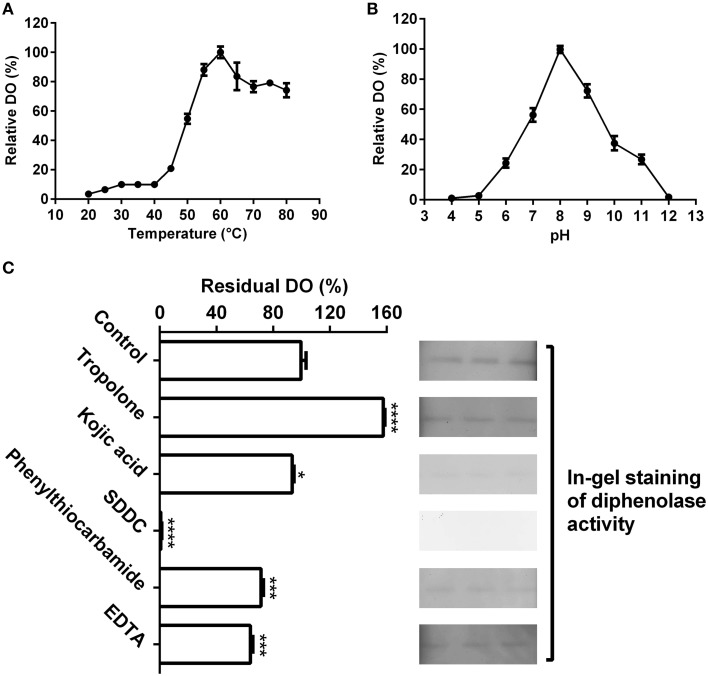
Figure 4.
Biochemical characterization of the diphenolase activity of the purified enzyme. (A) Optimal temperature of the diphenolase activity (DO) of the purified enzyme toward L-DOPA. (B) Optimal pH of the DO toward L-DOPA. (C) Effects of inhibitors (final concentration, 1 mM) on the DO toward L-DOPA. Left: DO was measured by spectrophotometer in the standard reaction mixture with or without (CK) inhibitors; Right: DO was detected by in-gel staining. After electrophoresis, each three lanes of the native PAGE gel with the purified enzyme (40 μL on every lane) were sliced out of the gel and incubated in 5 mM L-DOPA containing or not (CK) inhibitors for 60 min at 45°C. SDDC, sodium diethyldithiocarbamate. Data are from three independent experiments (mean ± SD). Statistical significance was analyzed by Student's t-test with the control; *P < 0.05; ***P < 0.001; ****P < 0.0001.