Abstract
Purpose
To determine if a fogging lens ameliorates accommodative effects driven by the closed-view design of the BHVI-EyeMapper (EM) instrument. We compared cycloplegic refraction and higher-order aberration measurements of the EM with those obtained with a fogging lens.
Methods
Twenty-six, young, participants (15F, 25 ± 5 years, range: 18–35 years, SE: +0.25 D and −3.50 D) with good ocular health were recruited. Five independent measurements of on- and off-axis refraction and higher-order aberrations were recorded across the horizontal visual field, under two conditions: non-cycloplegic measurements with +1.00 D fogging lens and cycloplegia, always in the same sequence. The contralateral eye was occluded during the measurements. Two drops of 1% Tropicamide delivered within 5 min facilitated cycloplegic measurements. All participants were refracted 30 min after installation of the second drop.
Results
Mean spherical equivalent measures of the non-cycloplegic condition were significantly more myopic than their cycloplegic counterparts (p < 0.05); approximately by 0.50 D centrally, increasing to 1.00 D towards the periphery. The horizontal astigmatic component, J180, demonstrated small but statistically significant differences between the test conditions. Differences were predominant for eccentricities greater than 30°, in both nasal and temporal meridians. The oblique astigmatic component, J45, was not significantly different between the test conditions. The primary spherical aberration coefficient C(4, 0) was significantly less positive for the non-cycloplegic state than its cycloplegic counterpart. This result held true across the entire horizontal visual field. The horizontal coma and trefoil coefficients C(3, 1) and C(3, 3) were not significantly different between the two conditions.
Conclusions
The use of +1.00 D fogging lens without cycloplegia did not provide complete relaxation of accommodation. The discrepancies between cycloplegic and non-cycloplegic EM measurements were found to be more pronounced for peripheral field angles than central measures, for both M and J180 components.
Keywords: Accommodation, Peripheral refraction and higher-order aberrations
Resumen
Objetivo
Determinar si una lente de miopización (fogging) mejora los efectos de acomodación impulsados por el diseño de campo cerrado del dispositivo BHVI-EyeMapper (EM). Comparamos las mediciones de la refracción ciclopéjica y las aberraciones de alto orden realizadas por el EM, con las obtenidas con una lente de miopización.
Métodos
Se reunió a veintiséis participantes jóvenes (15M, 25 ± 5 años, rango: 18–35 años, ES: +0,25 D y −3,50 D) con buena salud ocular. Se registraron cinco mediciones independientes de la refracción dentro y fuera de eje y de las aberraciones de alto orden a lo largo del campo visual, bajo dos situaciones diferentes: mediciones no ciclopéjicas con una lente de miopización de +1,00 D, y mediciones ciclopéjicas, siempre en la misma secuencia. El ojo contralateral fue ocluido durante la realización de las mediciones. La administración de dos gotas de Tropicamida 1%, en un plazo de cinco minutos, facilitó las mediciones ciclopéjicas. Todos los participantes fueron sometidos a refracción a los treinta minutos de la instilación de la segunda gota.
Resultados
Las mediciones del equivalente esférico de la situación no ciclopéjica reflejaron una miopía más considerable que las ciclopéjicas (p < 0,05); aproximadamente de 0,50 D centrales, incrementándose a 1,00 D hacia la periferia. El componente astigmático horizontal, J180, reflejó unas pequeñas diferencias, aunque estadísticamente significativas, entre las dos situaciones de la prueba. Las diferencias fueron predominantes para excentricidades superiores a 30°, tanto en los meridianos nasales como en los temporales. El componente astigmático oblicuo, J45, no reflejó una diferencia significativa entre ambas situaciones. El coeficiente de la aberración esférica primaria C(4, 0) fue considerablemente menos positivo en las situaciones no ciclopéjicas que en las ciclopéjicas. Este resultado mantuvo su validez a lo largo de todo el campo visual horizontal. Los coeficientes del coma horizontal y trefoil C(3, 1) y C(3, 3) no reflejaron una diferencia significativa entre ambas situaciones.
Conclusiones
El uso de una lente de miopización de +1,00 D, sin ciclopejía, no aporta una relajación completa de la acomodación. Las discrepancias entre las mediciones del dispositivo EM, con y sin ciclopejia, se revelaron más pronunciadas para los ángulos de campo periféricos que para los centrales, para ambos componentes M y J180.
Palabras clave: Acomodación, Refracción periférica y aberraciones de alto orden
Introduction
The actual mechanism of myopia genesis and its rate of progression are not clearly understood. Nevertheless, accumulated evidence from animal data suggests an involvement of the retinal image shell, as guided by peripheral refraction and off-axis higher-order aberrations (HOA).1, 2, 3 There is some clinical evidence in humans supporting the role of peripheral refraction in myopia progression.4, 5, 6, 7, 8, 9, 10 The emerging paradigm of the contribution of peripheral refraction to myopia development has triggered considerable interest in the peripheral optics of the eye.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 In the majority of these studies conventional, commercial instruments or procedures, have been modified to facilitate peripheral optics measurement, requiring repeated turning of the head or eye and tedious instrument re-alignment. For this reason, techniques that facilitate expedited measurements of peripheral refraction and/or aberrations have gained much attention in the last few years.26, 27, 28
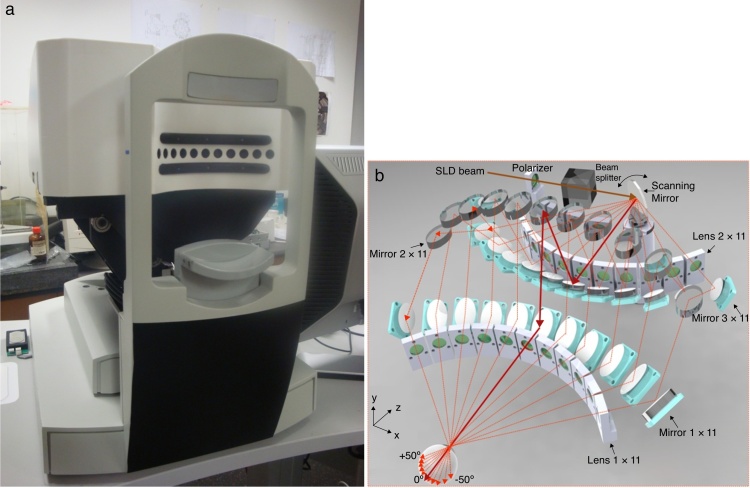
In response to the desire for automated peripheral refraction, the Brien Holden Vision Institute, Sydney, Australia developed the BHVI-EyeMapper (EM), a closed-view global aberrometer (Fig. 1). The EM performs fast (<0.5 s) refraction scans across 100° of the visual field in 10° steps, along horizontal, vertical and oblique visual field meridians, without the need for eye or head turn. Complete details of the working principles of the instrument can be found elsewhere.29 The EM has been previously validated against a conventional aberrometer (COAS-HD®, Wavefront Sciences, USA) in conjunction with Dynamic Stimulation Aberrometry (Optana®, Germany) and an open field autorefractor (Shin-Nippon NVision K5001®, Grand Seiko, Japan) for refraction measures obtained at distance and various near vergences (Bakaraju RC, et al. IOVS. 2012;53:ARVO E-Abstract 1354).30 Overall, the EM being influenced by its closed-view instrument design produces slightly ‘more negative’ results, about −0.50 D more myopic for distance refraction measures than those obtained with the Shin-Nippon autorefractor. However, results were in modest agreement with those obtained with COAS-HD aberrometer, another closed-view on-axis aberrometer. In the present study, we determined trends and relative differences in the on- and off axis refraction and HOA obtained using a fogging lens versus cycloplegia. Finally we sought to determine if, driven by its closed view instrument design, the fogging lens ameliorates accommodative effects on the refraction measurements.
Figure 1.
(a) The BHVI-EyeMapper (EM), Brien Holden Vision Institute, Sydney, Australia and (b) three-dimensional view describing the instrument design.
Methods
All the research procedures conformed to the regulations guided by the declaration of Helsinki. A local ethics committee (Bellberry, Adelaide, South Australia) approved the research protocol and the study was registered with the Australia New Zealand Clinical Trials Registry (#ACTRN12611001103954). Participants were recruited in response to an advertisement circulated via email to staff and eligible candidates on the patient database of the Brien Holden Vision Institute, Sydney, Australia. All participants were screened for suitability using routine ocular examination, which included auto-refraction (Nidek Tonoref II, Japan), subjective refraction, visual acuity and general ocular health examination with a slit-lamp biomicroscope and direct ophthalmoscope. A total of 26 ocular healthy participants (15 female) in the age range of 18–35 years (25 ± 5 years) with best-corrected visual acuity ≥20/20 in each test eye and a spherical equivalent refractive error between +0.25 D and −3.50 D were recruited for the study.
The EM is a closed-view aberrometer, designed to rapidly measure refractive errors and HOA of the eye over a wide range of viewing angles. The special feature of the EM is the use of numerous fixed optical components to steer the illumination and reflection beam to peripheral angles. A deflection system consisting of 33 mirrors and one scanning mirror was arranged to maintain equal optical path length while providing rapid scanning of the illumination beam across an angular range of up to ±50°, measuring peripheral refractive errors and HOA in discrete 10° steps along the selected meridian, including horizontal, vertical and some oblique meridians. One measurement scan (i.e. 11 positions) takes 0.45 s. The EM's working distance is 100 mm. The instrument is equipped with an internal back-illuminated fixation target mounted on a translation stage to facilitate accommodative response measurements. The software adjustable linear stage can be manoeuvred on a continuous scale to create a range of object vergences from +1.00 D (fogging) to −5.00 D (accommodative target).
Five independent wavefront measurements on the unaided eyes of each participant facilitated on- and off-axis, refraction and HOA, across the horizontal visual field under two test conditions. The measurements were always recorded in the following sequence: (1) no-cycloplegia with +1.00 D fogging; and (2) under cycloplegia with Tropicamide 1%. The fellow eye was always occluded with an eye patch. Two drops of 1% w/v Tropicamide within 5 min facilitated all cycloplegic measurements. For the cycloplegic test case, all participants were measured on the EM 30 min after the installation of the last drop. Under cycloplegia, visual acuity, auto-refraction (Nidek, Japan) and subjective refraction were assessed again. For all EM measurements, the illumination of the testing room was kept below 10 lux, to ensure the wavefront measures under ‘no-cycloplegia’ were greater than 4 mm pupil diameter. A Maltese cross with 8-arms designed to be telecentric in object space served as the fixation target. Each wavefront measurement provided a Hartmann–Shack image for further post-processing, the commercial camera software package HASO (Imagine Optics, France) was used to derive the spot locations. Once the spot locations were obtained, custom mathematical routines written in CVI/Lab windows were deployed to obtain slopes and fit them to Zernike polynomials derivatives. Refraction measures were derived as the Fourier vector components (M, J180 and J45) from the Zernike coefficients, as described elsewhere.31 In addition to the refraction measures, HOA up to 4th order in OSA standard were also obtained.
The refraction and HOA measurements were obtained at a 4 mm circular pupil diameter. In order to allow direct comparison of all data, i.e. cyclo versus non-cyclo, we did not consider elliptical pupils. Statistical analyses were performed using SPSS (version 18.0, Chicago, USA). Paired t-test was used to determine significant differences between cycloplegic and non-cycloplegic conditions at each visual field angle. Additional pairwise comparisons were performed between on-axis, cycloplegic and non-cycloplegic autorefraction obtained from EM, Nidek autorefractor and subjective refraction. Assuming the within participant SD to be 0.30 D, a sample size of 24 subjects was needed to detect a paired difference of 0.18 ± 0.3 D between the test conditions with alpha (α) set to 0.05 and statistical power to 80%. The sample size did not accommodate for dropouts, as the study needed only one participant visit.
Results
The means of the selected power vectors and aberration coefficients are presented as a function of retinal eccentricity (−50° to 50°) for both the test conditions. The sign convention used throughout the manuscript for the field angles is positive for nasal and negative for temporal fields. In the interest of brevity, only statistically significant and/or relevant HOA with respect to horizontal meridian are represented in figures and tables.
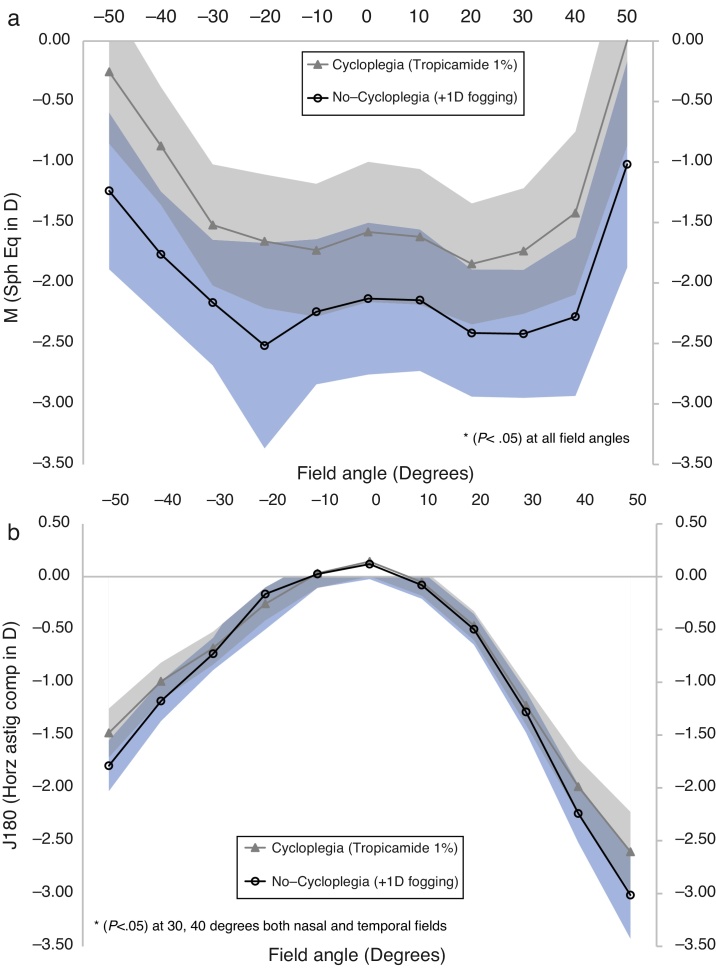
Fig. 2 showcases the differences in the measures of M and J180 as a function of horizontal visual field angle, for the two test conditions. At all field eccentricities, the mean spherical equivalent (M) measures obtained under the no-cycloplegia condition were significantly more myopic than under cycloplegia (p < 0.05). The differences were about 0.50 D centrally, increasing to about 1.00 D for peripheral field angles. Small (in the range of 0.25–0.50 D) yet statistically significant differences were found between the two test conditions, for J180 measures, particularly at peripheral field angles in the range of 30° to 40°. For the astigmatic component J45, there were no significant differences between the two conditions at any visual field angle.
Figure 2.
Refractive components M (spherical equivalent in diopters) as a function of horizontal visual field angle (in degrees) for two test conditions: (a) non-cycloplegic state with +1.00 D fogging lens and (b) cycloplegic state. The shaded areas indicate 95% confidence intervals. The symbol ‘*’ indicate statistical significant differences (p < 0.05) at the indicated field angles. Sign convention used was postive for nasal and negative for temporal field angles.
Table 1 compares three Zernike coefficients: horizontal coma C(3, 1), horizontal trefoil C(3, 3) and primary spherical aberration C(4, 0), as a function of visual field eccentricity for the two test conditions. As noted, there were no statistically significant differences in the horizontal coma C(3, 1), which held true across most field eccentricities, except for nasal 10° and 20° field angles. In general, small and statistically insignificant changes were found in horizontal trefoil, with exceptions at 40° and 50° nasal field eccentricities (p < 0.05). Primary spherical aberration C(4, 0) was found to be significantly less positive in magnitude for the non-cycloplegic state than the corresponding measures obtained under cycloplegia. This relationship held true across the entire horizontal visual field, except at the 30° nasal and temporal visual field angles, for which the differences became insignificant (p > 0.05). There were no statistically significant differences in the horizontal secondary astigmatism and horizontal tetrafoil across the field eccentricities (p > 0.05), except for temporal 30° for the former and temporal 30° and central field angles for the latter. There were no significant differences found between the two test conditions (p > 0.05), for the following on-axis variables (central field measures): oblique astigmatic component (J45), vertical coma C(3, −1), vertical trefoil C(3, −3), vertical tetrafoil C(4, −4) and vertical secondary astigmatism C(4, −2).
Table 1.
Aberration coefficients C(3, 1), C(3, 3) and C(4, 0) for 4 mm pupil diameter (in microns) as a function of horizontal visual field angle (in degrees) for cycloplegia and no-cycloplegia conditions. Sign convention for the field angles: positive for nasal and negative for temporal fields.
| Variables | Field angle | N | Cycloplegia |
No-cycloplegia |
p-Value | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| C(3, 1) Horizontal coma |
−50 | 26 | 0.407 | 0.190 | 0.381 | 0.164 | 0.082 |
| −40 | 26 | 0.250 | 0.100 | 0.246 | 0.096 | 0.514 | |
| −30 | 26 | 0.154 | 0.063 | 0.153 | 0.067 | 0.963 | |
| −20 | 26 | 0.105 | 0.049 | 0.117 | 0.100 | 0.560 | |
| −10 | 26 | 0.046 | 0.037 | 0.043 | 0.036 | 0.466 | |
| 0 | 26 | 0.000 | 0.028 | −0.004 | 0.029 | 0.282 | |
| 10 | 26 | −0.047 | 0.039 | −0.056 | 0.036 | 0.006 | |
| 20 | 26 | −0.089 | 0.037 | −0.102 | 0.039 | 0.003 | |
| 30 | 26 | −0.145 | 0.060 | −0.158 | 0.065 | 0.069 | |
| 40 | 26 | −0.261 | 0.120 | −0.254 | 0.122 | 0.371 | |
| 50 | 26 | −0.645 | 0.262 | −0.601 | 0.244 | 0.051 | |
| C(3, 3) Horizontal trefoil |
−50 | 26 | 0.118 | 0.109 | 0.102 | 0.080 | 0.130 |
| −40 | 26 | 0.043 | 0.070 | 0.041 | 0.054 | 0.747 | |
| −30 | 26 | 0.025 | 0.047 | 0.027 | 0.046 | 0.681 | |
| −20 | 26 | 0.013 | 0.042 | 0.042 | 0.121 | 0.286 | |
| −10 | 26 | 0.007 | 0.038 | 0.011 | 0.039 | 0.487 | |
| 0 | 26 | 0.001 | 0.036 | 0.005 | 0.036 | 0.334 | |
| 10 | 26 | −0.008 | 0.044 | −0.007 | 0.045 | 0.755 | |
| 20 | 26 | −0.011 | 0.054 | −0.016 | 0.056 | 0.392 | |
| 30 | 26 | 0.003 | 0.070 | 0.003 | 0.070 | 0.979 | |
| 40 | 26 | −0.036 | 0.096 | −0.011 | 0.084 | 0.001 | |
| 50 | 26 | −0.224 | 0.194 | −0.163 | 0.159 | 0.000 | |
| C(4, 0) Spherical aberration |
−50 | 26 | −0.046 | 0.045 | −0.058 | 0.048 | 0.083 |
| −40 | 26 | −0.003 | 0.024 | −0.014 | 0.030 | 0.011 | |
| −30 | 26 | −0.006 | 0.021 | −0.011 | 0.020 | 0.208 | |
| −20 | 26 | 0.006 | 0.021 | −0.014 | 0.063 | 0.096 | |
| −10 | 26 | 0.016 | 0.024 | 0.008 | 0.023 | 0.063 | |
| 0 | 26 | 0.024 | 0.022 | 0.017 | 0.024 | 0.002 | |
| 10 | 26 | 0.024 | 0.022 | 0.018 | 0.022 | 0.018 | |
| 20 | 26 | 0.015 | 0.021 | 0.007 | 0.028 | 0.013 | |
| 30 | 26 | −0.003 | 0.033 | −0.008 | 0.037 | 0.244 | |
| 40 | 26 | −0.029 | 0.047 | −0.040 | 0.046 | 0.095 | |
| 50 | 26 | −0.072 | 0.080 | −0.110 | 0.065 | 0.025 | |
All statistically significant p-values (i.e. p < 0.05) are italicized.
For the on-axis cycloplegic refraction, the Nidek autorefractor produced statistically significant lower values than the EM counterparts, for M (paired Δ = −0.13 ± 0.17 D, CI: −0.20 D to −0.06 D, p = 0.001) and J180 components (paired Δ = 0.05 ± 0.12 D, CI: 0.00–0.10 D, p = 0.047). However, all differences were clinically insignificant. The subjective refraction measures obtained under cycloplegia were also significantly lower than the corresponding EM measures, for M (paired Δ = −0.09 ± 0.21 D, CI: −0.17 D to 0.01 D, p = 0.044) and J180 components (paired Δ = 0.10 ± 0.16 D, CI: 0.04–0.17 D, p = 0.004), but again, were clinically insignificant. The oblique astigmatic component, J45, was not different when cycloplegic EM measures were compared with Nidek autorefractor and subjective refraction. When the non-cycloplegic measurements were considered, the EM reported clinically relevant higher levels of myopia than the Nidek autorefractor (paired ΔM = −0.41 ± 0.49 D, CI: −0.61 D to −0.22 D, p < 0.001) and subjective refraction (paired ΔM = −0.41 ± 0.52 D, CI: −0.62 D to −0.20 D, p < 0.001). No differences were observed for J180 and J45 components between the non-cycloplegic EM, Nidek and subjective measurements (Table 2).
Table 2.
The comparison of the mean on-axis refraction decomposed into three Fourier components (M, J180 and J45 in Diopters) obtained in the following conditions: (i) EM and Nidek autorefractor under complete cycloplegia; (ii) EM and Nidek autorefractor, under no-cycloplegia; (iii) EM and subjective refraction under cycloplegia and (iv) EM and subjective refraction under no-cycloplegia. All units are in Diopters.
| Variables | Field angle | N | EM (cycloplegia) |
Nidek (cycloplegia) |
p-Value | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| M | 0 | 26 | −1.58 | 1.51 | −1.45 | 1.50 | 0.001 |
| J180 | 0 | 26 | 0.14 | 0.37 | 0.09 | 0.33 | 0.047 |
| J45 | 0 | 26 | −0.04 | 0.17 | −0.03 | 0.19 | 0.693 |
| Variables | Field angle | N | EM (no-cycloplegia +1 D) |
Nidek (no-cycloplegia) |
p-Value | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| M | 0 | 26 | −2.13 | 1.63 | −1.72 | 1.36 | 0.000 |
| J180 | 0 | 26 | 0.12 | 0.37 | 0.07 | 0.34 | 0.170 |
| J45 | 0 | 26 | −0.03 | 0.19 | −0.01 | 0.16 | 0.573 |
| Variables | Field angle | N | EM (cycloplegia) |
Subjective (cycloplegia) |
p-Value | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| M | 0 | 26 | −1.58 | 1.51 | −1.49 | 1.43 | 0.044 |
| J180 | 0 | 26 | 0.14 | 0.37 | 0.04 | 0.27 | 0.004 |
| J45 | 0 | 26 | −0.04 | 0.17 | 0.00 | 0.15 | 0.065 |
| Variables | Field angle | N | EM (no-cycloplegia +1 D) |
Subjective (no-cycloplegia) |
p-Value | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| M | 0 | 26 | −2.13 | 1.63 | −1.72 | 1.36 | 0.000 |
| J180 | 0 | 26 | 0.12 | 0.37 | 0.06 | 0.28 | 0.052 |
| J45 | 0 | 26 | −0.03 | 0.19 | −0.01 | 0.14 | 0.419 |
Discussion
The goal of the current study was to identify if the measurements obtained by the EM are influenced by its closed-view design. Overall, in our sample of young adults, the mean spherical equivalent (M) measures in the non-cycloplegic condition were found to be significantly more myopic than their cycloplegic counterparts (p < 0.05). The average differences between the cycloplegic and non-cycloplegic states are in the order of half a diopter centrally, reaching up to one diopter, across the horizontal visual field. The results suggests that the use of a +1.00 D fogging lens during non-cycloplegic measurements did not provide sufficient relaxation of accommodation and certainly was also not comparable to the measures obtained for the cycloplegic state.
Querios et al.,32, 33 have demonstrated that successful relaxation of accommodation can be attained by the use of +2.00 D fogging spectacle lenses, while performing open-field central and peripheral refraction measurements. However, in contrast, in the current myopic participant sample, using the EM, we could not reproduce the claimed successful relaxation effects of accommodation. We offer two reasons for this lack of agreement. Firstly, the magnitude of the fogging lens used in the current study was exactly half of that proposed by Querios et al. As mentioned briefly in “Methods” section, the prototype EM can facilitate a six diopter range of defocus via its internal, telecentric, Badal system (−5.00 D to +1.00 D). This range of defocus limited our choice of fogging to a maximum of +1.00 D, without disturbing the telecentricity of the optical system. We plan to rectify this limitation by replacing the current Badal system with that of a much larger dioptric range for future studies. Secondly, considering the closed-view design of the EM (as opposed to the measures reported with open-field autorefractor), the very proximity of this instrument potentially created a weak stimulus for accommodation. Nevertheless, it is important to note that Querios and colleagues reported in their sub-group analysis that the paired differences between the cycloplegic state and the non-cycloplegic state with fogging lenses was significantly different only in the myopic group. Furthermore, contrary to our results, they reported lower mean spherical equivalent myopic refractive error with use of fogging lenses than those achieved with cycloplegia, indicating that either cycloplegia was ineffective or the measurement data were affected by the use of a fogging lens itself.
Carkeet et al.,34 investigated the effect of cycloplegia on ocular aberrations in young adults using Technolas Zywave aberrometer (Bausch and Lomb, USA) and found small yet statistically significant differences between higher order measures (3rd and 5th order) between cycloplegic and non-cycloplegic states. Hiraoka et al.,35 confirmed similar results after assessing the effect of cycloplegia on ocular aberrations in children using the Topcon Hartmann-Shack aberrometer (KR-9000PW, Topcon, Tokyo, Japan) and 1% Atropine. Our results, reported from a custom-built instrument, are in agreement with these previous reports. However, it is worth noting that unlike in Hiraoka et al., we used 1% Tropicamide, for a shorter duration of cycloplegic effect. Further, it is important to note that the previous studies examined the effects of cycloplegia on the on-axis HOA while the current study extends this to horizontal peripheral visual field, encompassing −50° to 50°.
Although small, it is interesting to note that there were significant differences between the Nidek autorefractor and EM measures even under complete cycloplegia. Such discrepancies in measurements could be plausibly explained by the differences in the measurement techniques. The EM is based on Hartmann–shack aberrometry principles in which a complete circular region of interest is considered for obtaining refraction measurements, while the Nidek autorefractor analyses projected infrared rings that only consider an annular region of the pupil. Further, it is worth noting that the EM measures were computed using Seidel defocus (i.e. paraxial curvature matched measures that includes defocus and primary spherical aberration) unlike the Nidek.
It can be argued that the use of a single power fogging lens (+1 D) for testing various degrees of myopic participants in the study could be a limitation, as the fogging lens could have resulted in a slightly different level of accommodation control for different magnitudes of myopia. We chose to use one identical test case scenario across the experiment to reduce the possible effects of other confounding variables.
Overall, our results suggest that the refraction values from the EM obtained for non-cycloplegic conditions could be slightly more myopic than their cycloplegic counterparts, despite the use of a +1.00 D fogging lens while performing the measurement. This shift can principally attributed to instrument-driven proximal accommodation. While performing subjective refraction, using the objective EyeMapper output as a start point, it is important to bear in mind that the start point could be geared towards more myopia or less hyperopia and appropriate subjective fogging techniques are needed for an optimal refraction endpoint. The EM used here was a prototype. The next generation capable of providing an increased dioptric range to facilitate greater levels of fogging is now under development. Further work may be required to assess whether the use of stronger fogging lenses would ameliorate accommodation effects due to the closed-view nature of the instrument, in particular, in studies that involve even younger participants for whose refraction measurements, accommodation control is much more crucial.
Conclusion
The use of +1.00 D fogging lens did not provide complete relaxation of accommodation which observation was clearly demonstrated when compared with the measurements produced under cycloplegia. Data from older individuals in the study confirm that proximal accommodation due to the closed view design of the EM plays an important role while performing refraction measurements. Accommodation affects the peripheral refraction more than central refraction for both, M and J180. A stronger fogging lens may be needed in vision studies involving younger populations where accommodation control can be crucial.
Conflicts of interest
Some of the authors are inventors on the patents that relate to the BHVI-EyeMapper: C. Fedtke (AU2011902736), K. Ehrmann (WO 2008/116270 A1, AU2011902736), D. Falk (AU2011902736), B. Holden (WO 2008/116270 A1).
Acknowledgements
The authors would like to acknowledge the clinical team (Ms. Chung, Mr. Ozkan, Ms. Robertson and Ms. Crompton) and the database management team (Dr. Naduvilath and Ms. Laarakkers) for their invaluable support to run the study at the Clinical Trial Research Centre, Brien Holden Vision Institute, Sydney, Australia. Authors would like to extend their thanks to Dr. Flanagan for reviewing the manuscript.
References
- 1.Mutti D.O., Sholtz R.I., Friedman N.E., Zadnik K. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41:1022–1030. [PubMed] [Google Scholar]
- 2.Hung L.F., Ramamirtham R., Huang J., Qiao-Grider Y., Smith E.L., 3rd Peripheral refraction in normal infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2008;49:3747–3757. doi: 10.1167/iovs.07-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith E.L., 3rd Prentice Award Lecture 2010: a case for peripheral optical treatment strategies for myopia. Optom Vis Sci. 2011;88:1029–1044. doi: 10.1097/OPX.0b013e3182279cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X., Sankaridurg P., Donovan L. Characteristics of peripheral refractive errors of myopic and non-myopic Chinese eyes. Vis Res. 2010;50:31–35. doi: 10.1016/j.visres.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Mutti D.O., Hayes J.R., Mitchell G.L. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48:2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charman W.N., Radhakrishnan H. Peripheral refraction and the development of refractive error: a review. Ophthalmic Physiol Opt. 2010;30:321–338. doi: 10.1111/j.1475-1313.2010.00746.x. [DOI] [PubMed] [Google Scholar]
- 7.Sankaridurg P., Donovan L., Varnas S. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optom Vis Sci. 2010;87:631–641. doi: 10.1097/OPX.0b013e3181ea19c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sankaridurg P., Holden B., Smith E. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Invest Ophthalmol Vis Sci. 2011;52:9362–9367. doi: 10.1167/iovs.11-7260. [DOI] [PubMed] [Google Scholar]
- 9.Walline J.J., Greiner K.L., McVey M.E., Jones-Jordan L.A. Multifocal contact lens myopia control. Optom Vis Sci. 2013;90:1207–1214. doi: 10.1097/OPX.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 10.Walline J.J., Lindsley K., Vedula S.S., Cotter S.A., Mutti D.O., Twelker J.D. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2011;(12.) doi: 10.1002/14651858.CD004916.pub3. Art. No.: CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes-Ferreira D., Ribeiro C., Maia R. Peripheral myopization using a dominant design multifocal contact lens. J Optom. 2011;4:14–21. [Google Scholar]
- 12.Atchison D.A., Pritchard N., White S.D., Griffiths A.M. Influence of age on peripheral refraction. Vis Res. 2005;45:715–720. doi: 10.1016/j.visres.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Atchison D.A., Pritchard N., Schmid K.L. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vis Res. 2006;46:1450–1458. doi: 10.1016/j.visres.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Charman W.N., Jennings J.A. Longitudinal changes in peripheral refraction with age. Ophthalmic Physiol Opt. 2006;26:447–455. doi: 10.1111/j.1475-1313.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 15.Charman W.N., Mountford J., Atchison D.A., Markwell E.L. Peripheral refraction in orthokeratology patients. Optom Vis Sci. 2006;83:641–648. doi: 10.1097/01.opx.0000232840.66716.af. [DOI] [PubMed] [Google Scholar]
- 16.Atchison D.A., Scott D.H., Charman W.N. Measuring ocular aberrations in the peripheral visual field using Hartmann–Shack aberrometry. J Opt Soc Am A Opt Image Sci Vis. 2007;24:2963–2973. doi: 10.1364/josaa.24.002963. [DOI] [PubMed] [Google Scholar]
- 17.Calver R., Radhakrishnan H., Osuobeni E., O’Leary D. Peripheral refraction for distance and near vision in emmetropes and myopes. Ophthalmic Physiol Opt. 2007;27:584–593. doi: 10.1111/j.1475-1313.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 18.Berntsen D.A., Mutti D.O., Zadnik K. Validation of aberrometry-based relative peripheral refraction measurements. Ophthalmic Physiol Opt. 2008;28:83–90. doi: 10.1111/j.1475-1313.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 19.Radhakrishnan H., Charman W.N. Peripheral refraction measurement: does it matter if one turns the eye or the head? Ophthalmic Physiol Opt. 2008;28:73–82. doi: 10.1111/j.1475-1313.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- 20.Mathur A., Atchison D.A., Charman W.N. Myopia and peripheral ocular aberrations. J Vis. 2009;9:15 11–1512. doi: 10.1167/9.10.15. [DOI] [PubMed] [Google Scholar]
- 21.Whatham A., Zimmermann F., Martinez A. Influence of accommodation on off-axis refractive errors in myopic eyes. J Vis. 2009;9:1411–1413. doi: 10.1167/9.3.14. [DOI] [PubMed] [Google Scholar]
- 22.Atchison D.A., Mathur A., Read S.A. Peripheral ocular aberrations in mild and moderate keratoconus. Invest Ophthalmol Vis Sci. 2010;51:6850–6857. doi: 10.1167/iovs.10-5188. [DOI] [PubMed] [Google Scholar]
- 23.Kang P., Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optom Vis Sci. 2011;88:476–482. doi: 10.1097/OPX.0b013e31820f16fb. [DOI] [PubMed] [Google Scholar]
- 24.Tabernero J., Ohlendorf A., Fischer M.D., Bruckmann A.R., Schiefer U., Schaeffel F. Peripheral refraction profiles in subjects with low foveal refractive errors. Optom Vis Sci. 2011;88:E388–E394. doi: 10.1097/OPX.0b013e31820bb0f5. [DOI] [PubMed] [Google Scholar]
- 25.Kwok E., Patel B., Backhouse S., Phillips J.R. Peripheral refraction in high myopia with spherical soft contact lenses. Optom Vis Sci. 2012;89:263–270. doi: 10.1097/OPX.0b013e318242dfbf. [DOI] [PubMed] [Google Scholar]
- 26.Tabernero J., Schaeffel F. Fast scanning photoretinoscope for measuring peripheral refraction as a function of accommodation. J Opt Soc Am A Opt Image Sci Vis. 2009;26:2206–2210. doi: 10.1364/JOSAA.26.002206. [DOI] [PubMed] [Google Scholar]
- 27.Wei X., Thibos L. Design and validation of a scanning Shack Hartmann aberrometer for measurements of the eye over a wide field of view. Opt Express. 2010;18:1134–1143. doi: 10.1364/OE.18.001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaeken B., Lundstrom L., Artal P. Fast scanning peripheral wave-front sensor for the human eye. Opt Express. 2011;19:7903–7913. doi: 10.1364/OE.19.007903. [DOI] [PubMed] [Google Scholar]
- 29.Fedtke C., Ehrmann K., Falk D., Bakaraju R.C., Holden B.A. The BHVI-EyeMapper: peripheral refraction and aberration profiles. Optom Vis Sci. 2014;91:1199–1207. doi: 10.1097/OPX.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedtke C., Ehrmann K., Falk D., Holden B.A. Validation of a quasi real-time global aberrometer: the EyeMapper. Proc SPIE. 2012;8209:1–7. [Google Scholar]
- 31.Salmon T.O., West R.W., Gasser W., Kenmore T. Measurement of refractive errors in young myopes using the COAS Shack–Hartmann aberrometer. Optom Vis Sci. 2003;80:6–14. doi: 10.1097/00006324-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Queiros A., Gonzalez-Meijome J., Jorge J. Influence of fogging lenses and cycloplegia on open-field automatic refraction. Ophthalmic Physiol Opt. 2008;28:387–392. doi: 10.1111/j.1475-1313.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- 33.Queiros A., Jorge J., Gonzalez M. Influence of fogging lenses and cycloplegia on peripheral refraction. J Optom. 2009;2:83–89. [Google Scholar]
- 34.Carkeet A., Velaedan S., Tan Y.K., Lee D.Y., Tan D.T. Higher order ocular aberrations after cycloplegic and non-cycloplegic pupil dilation. J Refract Surg. 2003;19:316–322. doi: 10.3928/1081-597X-20030501-08. [DOI] [PubMed] [Google Scholar]
- 35.Hiraoka T., Miyata K., Nakamura Y. Influences of cycloplegia with topical atropine on ocular higher-order aberrations. Ophthalmology. 2013;120:8–13. doi: 10.1016/j.ophtha.2012.07.057. [DOI] [PubMed] [Google Scholar]