FIGURE 10.
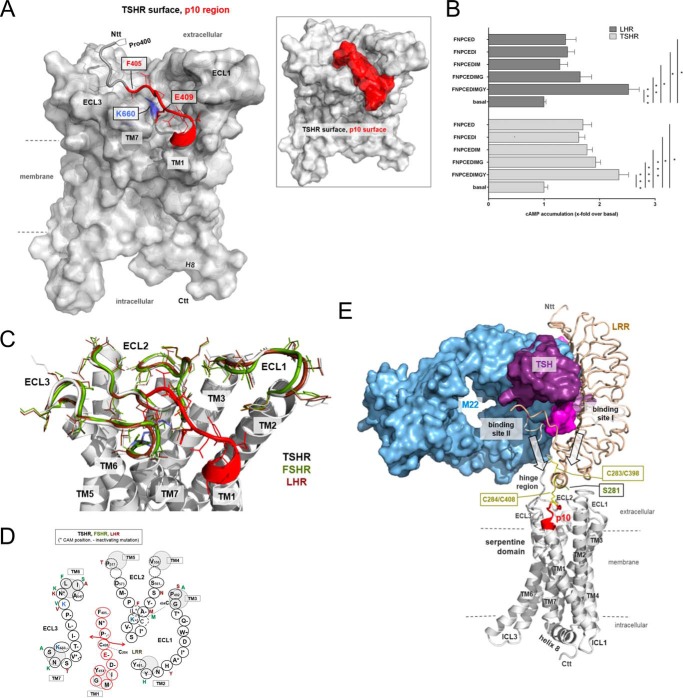
Structural homology model of the TSHR serpentine domain including the p10 region. A, the 7TM structure of the TSHR with the p10 region (red, backbone ribbon tube, side chains from Phe405–Tyr414), which equates in the TSHR exactly the transition between the extracellular HR and the TM1, is represented as a model based on crystal structures of homologous GPCRs (see “Experimental Procedures”). Ntt, N-terminal tail; Ctt, C-terminal tail. Inset: the surfaces of the 7TM and the p10 region are highlighted, showing that the flexibility of this fragment between the ECLs is spatially restricted by steric constraints. B, COS-7 cells transfected with TSHR and LHR were incubated with shortened p10 (1 mm) and p10 as positive control. cAMP levels of mock-transfected cells were 2.1 ± 1.1 nm/well. Data are means ± S.E. of three experiments performed in triplicate. *, p < 0.05, **, p < 0.01, ***, p < 0.001 (paired Student's t test). C, the positioning of the p10 region is shown in the overlay of the TSHR, LHR, and FSHR. Based on this model, the amino acid interface of p10 and its binding pocket is shown in D. Several positions of this interface were mutated and functionally tested. Positions marked with + lead to receptor activation, and positions marked with − lead to receptor inactivation. For references, see the TSHR database Sequence-Structure-Function-Analysis of Glycoprotein Hormone Receptors (46). E, the complex model of TSHR (white, backbone ribbon) with bound TSH and the activating antibody M22 visualizes a potential arrangement and the principle mechanism of GPHR activation. For comparison of binding sites, the LRR domain of TSHR complexed with the activating antibody M22 (9) was superimposed with the TSHR LRR domain/TSH model. The entire complex is derived by the arrangement of available structural information on GPHRs and GPCRs (see “Experimental Procedures”). ICL, intracellular loop.