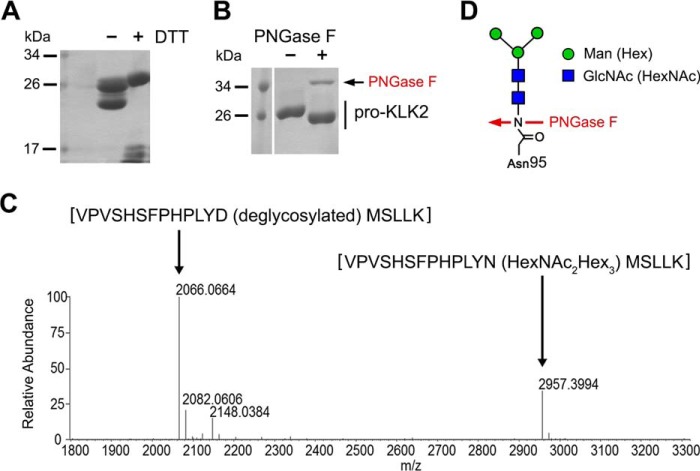
FIGURE 1.
Analysis of KLK2 glycosylation. A, enterokinase-activated KLK2 from Leishmania expression (LEXSY) was analyzed under non-reducing and reducing conditions. Clipped products migrated as a single band under non-reducing conditions. Intact and clipped forms, which form fragments smaller than 17 kDa under reducing conditions (+DTT), were not separable and appeared as a single peak in gel filtration. B, removal of N-glycans from LEXSY-KLK2 with PNGase F (36 kDa) resulted in a shift of the apparent molecular mass by about 1 kDa. C, trypsin-digest mass spectrometry confirmed partially deglycosylated LEXSY-KLK2 by PNGase F under non-denaturing conditions. The deconvoluted mass spectrum is shown, and the indicated masses refer to uncharged molecules. The peak at 2957 was identified by sequencing and subsequent modification search as VPVSHSFPHPLYN-(Hex3HexNAc2)-MSLLK. The signal at 2066 represents the corresponding deglycosylated peptide (VPVSHSFPHPLYD-(deglycosylated)-MSLLK), with the expected core glycan mass of 891 Da. D, N-linked core glycans at Asn-95 of LEXSY KLK2, consisting most likely of two N-acetylglucosamine and three mannose units. PNGase F cleaves the N-glycan and generates an Asp-95.