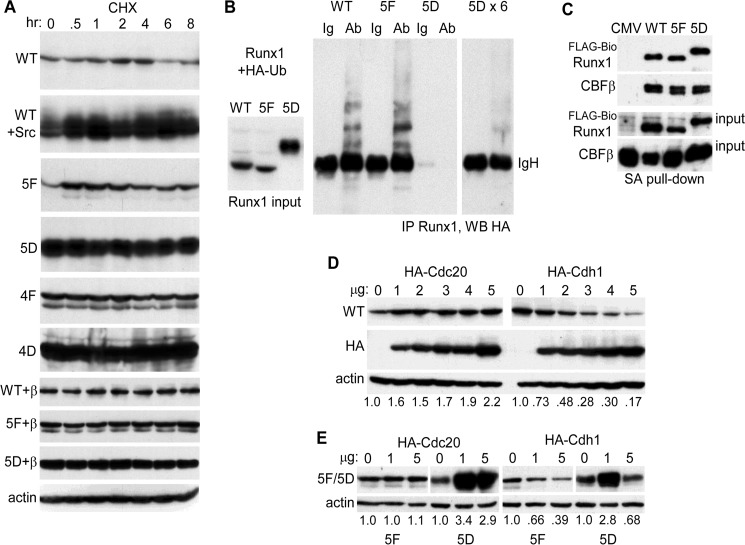
FIGURE 3.
A, 293T cells in 6-well dishes were transfected with 1 μg of CMV-Runx1 (WT), 0.25 μg of WT + 1 μg of CMV-Src; 1 μg of CMV-5F, CMV-5D, CMV-4F, or CMV-4D; 1 μg of CMV-WT + 1 μg of CMV-CBFβ, 5F + CBFβ, or 5D + CBFβ. CHX was added for 0.5–8 h, and total cellular proteins from equal numbers of cells were then evaluated for Runx1 isoform expression by Western blotting. Actin expression was assessed on similarly cultured 293T cells exposed to CHX. B, 293T cells in 10-cm dishes were transfected with 2 μg of CMV-WT, 1 μg of CMV-5F, or 0.4 μg of CMV-5D with 3 μg of CMV-HA-ubiquitin (HA-Ub). Extracts prepared 2 days later were subjected to Runx1 Western blotting, with 1/12th volume of the 5D extract compared with the WT or 5F extracts loaded to equalize expression (left). In addition 0.5 mg of total cell lysates were subjected to rabbit anti-Runx1 immunoprecipitation followed by anti-HA (16B12) Western blotting, with 1/12th (center) or 1/2 (right) of the 5D immunoprecipitate loaded relative to WT or 5F. The position of immunoglobulin heavy chain (IgH) is shown. Ab, antibody. C, total cellular proteins from 293T cells in 10-cm dishes transfected with 1.5 μg of CMV-CBFβ, 1.5 μg of EF1α-birA, and 3 μg of EF1α-FLAG-BioWT, 3 μg of EF1α-FLAG-Bio5F, or 10 ng of EF1α-FLAG-Bio5D were subjected to streptavidin-agarose pulldown followed by Western blotting of 2.5% input or pulldown samples using Runx1 and CBFβ antisera. D, 293T cells in 6-well dishes were transfected with 2 μg of Runx1 and 0–5 μg of HA-Cdc20 or HA-Cdh1 expression vectors. Total cellular proteins were then analyzed by Western blotting using Runx1, HA (Y-11), and β-actin antibodies. The relative intensities of the Runx1 bands, normalized to β-actin expression, are shown below each lane. E, 293T cells were transfected with 1 μg of 5F or 0.5 μg of 5D along with 0, 1, or 5 μg of HA-Cdc20 or HA-Cdh1 expression vectors, followed by Western blot analysis using Runx1 or β-actin antibodies.