Figure 3.
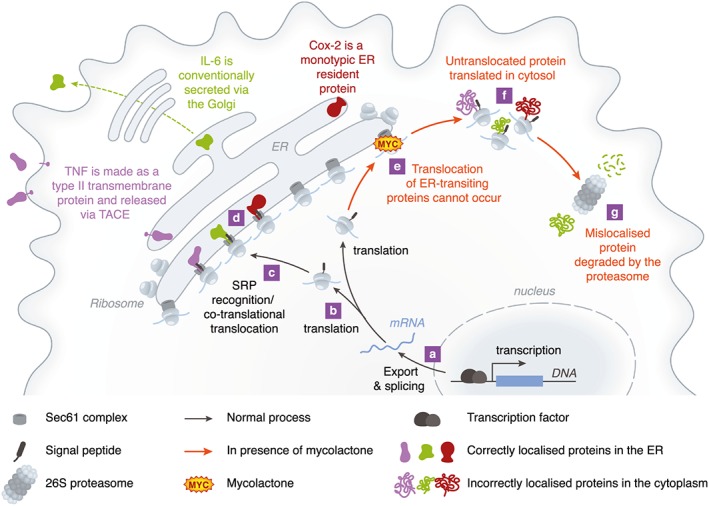
Mycolactone inhibits the co‐translational translocation of proteins via Sec61. Sec61‐dependent, ER‐transiting proteins are derived from mRNAs (a) and usually have a signal peptide sequence at the amino terminus (b). Once this is formed, translation pauses and the signal peptide is recognized by the SRP (not shown), which transports the ribosome/mRNA/nascent peptide complex to the Sec61 complex at the ER membrane (c). The hydrophobic signal peptide interacts with Sec61 and translation continues, directly into the ER lumen (d); a process further facilitated by chaperones such as BiP (not shown). A similar process occurs for transmembrane proteins (TNF), monotypic proteins (COX‐2) and conventionally secreted proteins (IL‐6). In the presence of mycolactone, translocation cannot occur (e) so translation takes place in the cytoplasm instead (f), and the proteins, recognized by the cell as being in the wrong compartment, are destroyed almost immediately by the 26S proteasome (g). This means that induced proteins can never be detected, and constitutive proteins are lost from the cell as they cannot be replaced during normal protein recycling.
