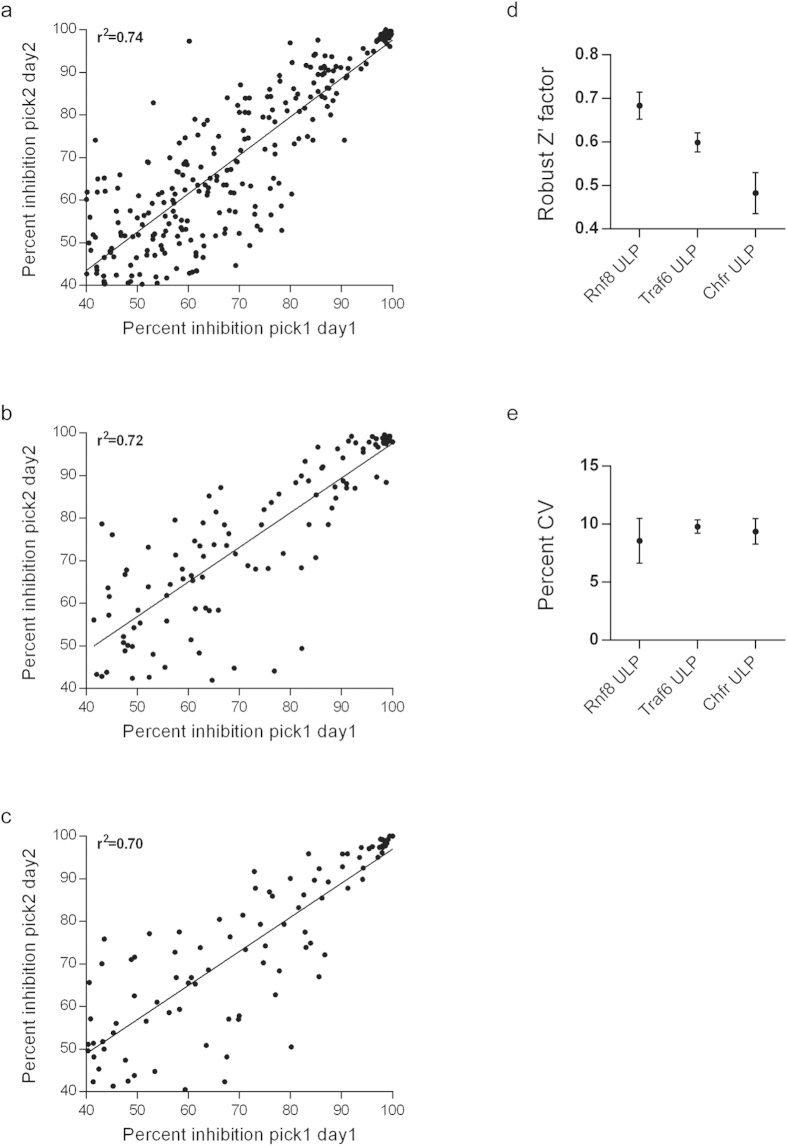
Figure 3. Validation of the ULP method.
(a–c) (a) Rnf8, (b) Traf6 and (c) Chfr assay-ready cell bank validated with AstraZeneca test set comprised of 9,846 compounds to determine data reproducibility. Cryopreserved assay-ready cells were dispensed into compound-ready 384-well plates on two separate days with different compound well locations (pick1 or pick2). Firefly luciferase activity was determined following 20-hour incubation. Data shown for compounds with ≥40% activity on both occasions. (d) Z’ factors of ULP assays (Rnf8 ULP −0.68 ± 0.02 SEM; Traf6 ULP −0.60 ± 0.01 SEM; Chfr ULP −0.48 ± 0.03 SEM). (e) Coefficient of variation (CV) of ULP assays (Rnf8 ULP −8.6% ± 1.1 SEM; Traf6 ULP −9.8% ± 0.3 SEM; Chfr ULP −9.4 %± 0.6 SEM).