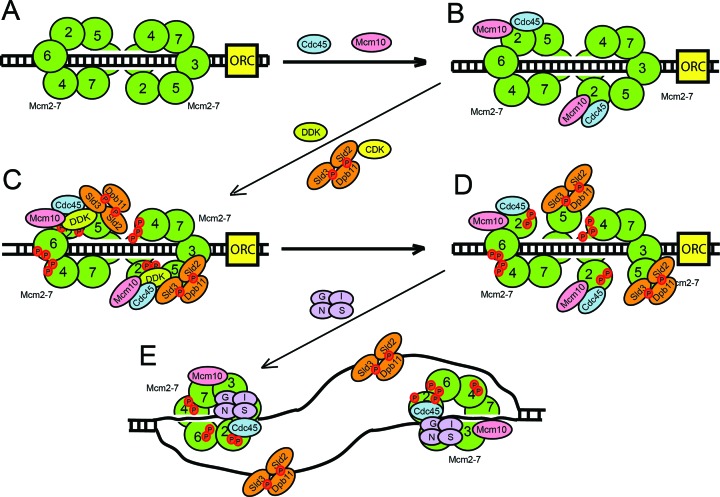
Figure 7.
Mcm10 regulates the assembly of the CMG complex and DNA origin melting. (A) Mcm2–7 complex is loaded as a double hexamer to encircle dsDNA during late M and G1 phase. (B) In early S phase, Mcm10 interacts with the Mcm2–7 complex and recruits Cdc45 to Mcm2–7. Five molecules of Mcm10 may bind to each Mcm2–7, but only one Mcm10 is shown for clarity. (C) Mcm10 stimulates DDK phosphorylation of Mcm2. Sld3 also stimulates DDK phosphorylation of Mcm2, and Sld3 can also recruit Cdc45 in a manner that depends upon DDK phosphorylation of Mcm2. Sld3 has been recently shown to form a heterotetramer with Sld7 (68), but just one Sld3 molecule is shown for clarity. S-phase cyclin dependent kinase (CDK) phosphorylates Sld2 and Sld3 and these phosphorylated proteins bind to Dpb11 to form the ternary complex Sld3–Sld2–Dpb11 that binds to the Mcm2–7 complex. The Sld3–Sld2–Dpb11 ternary complex blocks the interaction between GINS and Mcm2–7. (D) Phosphorylation of Mcm2 by DDK opens the Mcm2–Mcm5 gate, allowing the extrusion of ssDNA from the central ring of Mcm2–7 complex. (E) Sld3-Sld2-Dpb11 binds to ssDNA and GINS binds to the Mcm2–7-Cdc45 complex, releasing Mcm10 from Cdc45 and forming the closed Cdc45–Mcm2–7–GINS active helicase complex that encircles ssDNA.