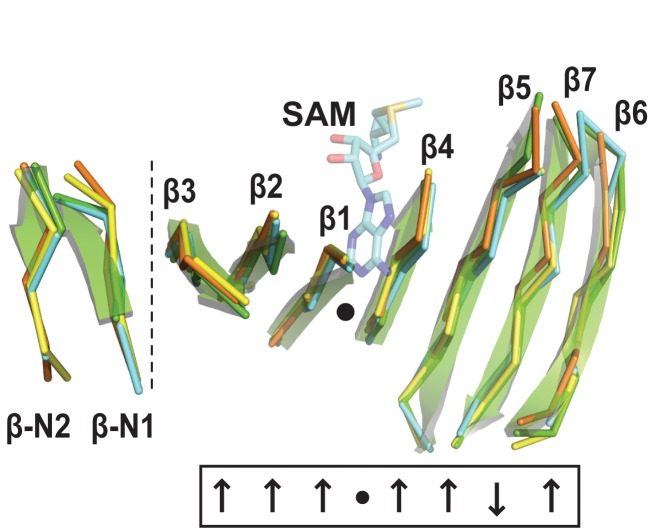
Figure 1.
The conserved β-sheet core of the 16S rRNA (m1A1408) methyltransferase family. Class I methyltransferases possess a structurally conserved, seven-stranded β-sheet (β1 to β7) with a central topological switch point (black dot) that creates the SAM-binding pocket. The conserved β-sheet core of the 16S rRNA (m1A1408) methyltransferases is extended by a short N-terminal extension (β-N1 and β-N2, separated by the vertical dotted line). Structures shown are NpmA (cyan; PDB code 3MTE), KamB (orange; 3MQ2), Kmr (yellow; 4RWZ) and CacKam from C. acidiphilia (green cartoon; this study). The bound SAM (semi-transparent cyan sticks) is from the crystal structure of the NpmA–SAM complex (3MTE).