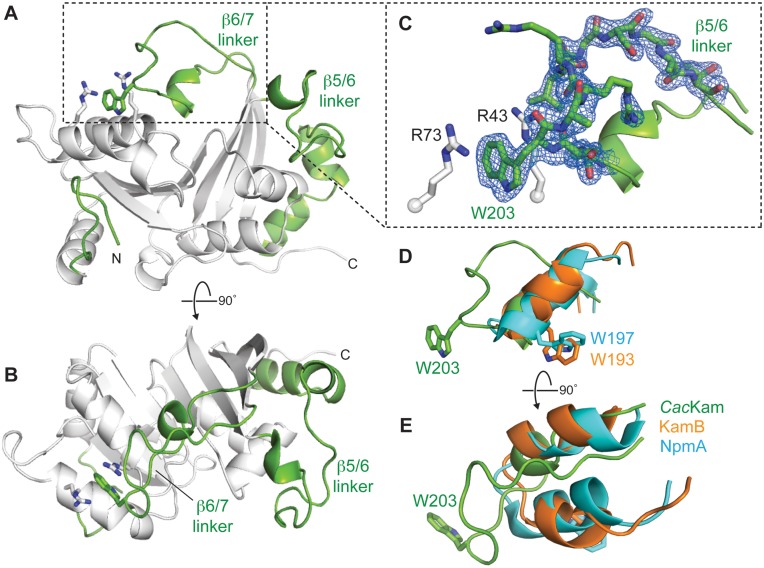
Figure 2.
The crystal structure of apo CacKam methyltransferase reveals a novel conformation of the functionally critical β6/7 linker. (A and B) Two orthogonal views of the 1.7-Å resolution crystal structure of apo CacKam highlighting (green) the three regions which augment the Class I methyltransferase fold at the N-terminus (N), and between β-strands 5 and 6 (β5/6 linker), and 6 and 7 (β6/7 linker). Interacting residues stabilizing the novel β6/7 linker conformation, R43/ R73 (white sticks) and W203 (green sticks), are also shown. (C) Zoomed in view of the CacKam β6/7 linker and the cation-π-cation interaction of residues R73/W203/R43. Residues S201-T212 of the β6/7 linker are shown in 2Fo − Fc electron density contoured at 1.0σ. (D and E) Two orthogonal views comparing the β6/7 linker structure of CacKam (green), NpmA (cyan) and KamB (orange), shown in the same orientation as the view of panel A. Functionally critical NpmA (W197) and KamB (W193) tryptophan residues equivalent to CacKam W203 are also shown.