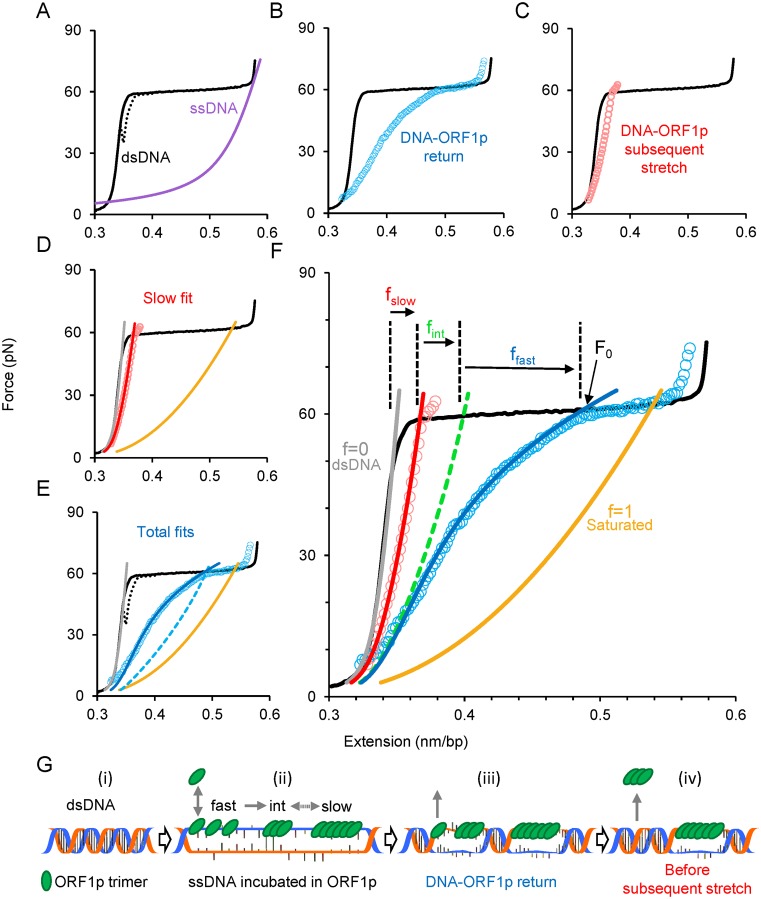
Figure 5.
Single molecule analysis reveals and quantifies three populations of ORF1p bound to ssDNA. (A) Stretch (solid black line) and return (dashed black line) curves of a dsDNA molecule in the absence of ORF1p. Purple line is the force-extension curve of an ssDNA. (B) Return of the ORF1p-DNA complex (blue circles) after incubating an overstretched dsDNA for 360 s in 2 nM ORF1p (111p). (C) Subsequent stretch (red circles) of the 111p–DNA complex shown in (C). (D) Quantifying the 111p-bound ssDNA fraction (f) bound by ORF1p exhibiting slow dissociation kinetics. The subsequent stretch is fit (red line, f = fslow) to a linear combination of dsDNA (gray line, f = 0) and the 111p-saturated ssDNA (solid gold, f = 1) curves. (E) Quantification of the total fraction (fT) of 111p-bound ssDNA. Dashed blue curve is the linear combination intersecting the force (F0) at which the 111p–DNA complex begins to approach the force regime below the melting plateau, which yields fT. Return data is fit by allowing ffast to vary with force in order to correct for the rapid 111p dissociation during the return to find ffast and fint. (F) Summary of the analysis method. The green dashed line is the summed linear combination of fslow and fint. (G) Schematic of the model used to interpret the data from (A–F), representing a single event from each kinetic class of ssDNA-bound ORF1p (not to scale and does not reflect the total fraction in each state). DsDNA (i) is initially stretched in the optical tweezers to convert it into ssDNA through force-induced melting. The stretched ssDNA is incubated in the solution containing ORF1p (ii) resulting in a combination of three types of bound protein: fast, intermediate and slow. The bound fast component equilibrates quickly with the protein in solution, but is also converted into small oligomers (intermediate component) and then into large oligomers (slow component) on ssDNA (ii). As the DNA–protein complex is released, the fast component quickly dissociates (iii). When the DNA is completely relaxed, just before the subsequent stretch, the intermediate component dissociates, leaving only the slow component bound (iv). (See also Supplementary Methods and Supplementary Figure S3).