Fig. 1.
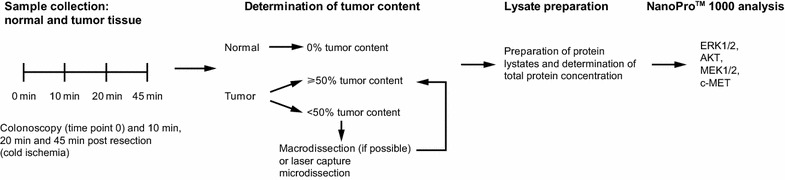
Study workflow indicating cold ischemia time points, quality control of samples, and subsequent proteomic analysis. Patient samples were collected at time point 0 and subjected to cold ischemia. Subsequently, the tumor content of samples was adjusted to ≥50 % and protein lysates were prepared for further proteomic analysis
