Figure 1.
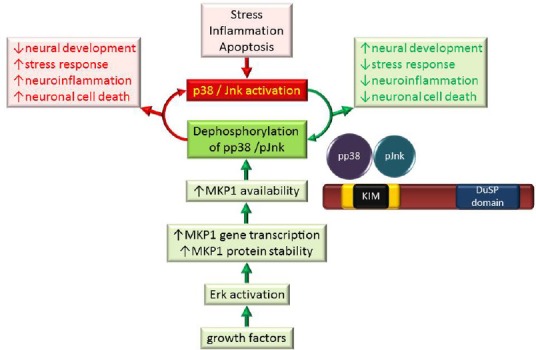
MKP1 attenuates the detrimental effects of p38 and Jnk kinases.
Cellular stress, inflammation or apoptosis may lead to the phosphorylation and activation of p38 and Jnk MAP kinases which contribute to decreased neural development, increased stress response, increased neuroinflammation and increased neuronal cell death in the nervous system (red box). Growth factor signalling leads to Erk activation which stimulates gene transcription and increases MKP1 protein stability and availability. The resulting increase in MKP1 activity leads to a decrease in p38 and Jnk activity, contributing to improvements in neural development, decreased neuroinflammation and reduced neuronal cell death (green box). Activated MAP kinases are bound by MKP1's kinase interaction motif (KIM) with decreasing preference (p38 > Jnk > Erk) and dephosphorylated by the dual specificity phosphatase (DuSP) domain. Erk: Extracellular signal-regulated kinase; Jnk: c-Jun N-terminal kinase; MAP: mitogen-activated protein; MKP1: mitogen-activated protein kinase phosphatase 1; p38: 38 kDa protein; pJnk: p-c-Jun N-terminal kinase; pp38: 38-kDa phosphorylated protein.
