Figure 1.
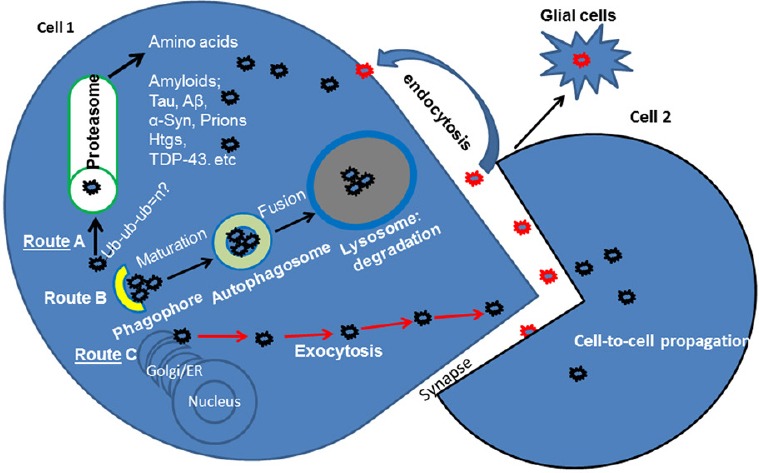
Protein propagation and secretion in neurodegeneration.
Intracellular (cell 1) accumulation of pathogenic or misfolded proteins triggers quality control clearance mechanisms. Route A–Protein can be ubiquitinated (Ub: Ubiquitin) and degraded via the proteasome. Route B–Autophagy is a major bulk degradation pathway. Accumulating proteins are first sequestered into a phagophore, which matures into a double-membrane autophagosome and fuses with the lysosome that contains digestive enzymes. If proteins are not degraded due to failure of the proteasome or autophagy, they may be secreted (Route C via exocytosis) into the synapse. Toxic proteins in the synaptic cleft may cross to (cell 2) an adjacent cell, resulting in trans-synaptic protein propagation. Extracellular or secreted proteins may be re-routed via the endosomal system and re-internalized into Cell 1 or destroyed by immune responses via activated microglia. Aβ: Amyloid beta; α-Syn: alpha-Synuclein; ER: endoplasmic reticulum; TDP-43: transactive response DNA binding protein 43 kDa.
