Figure 2.
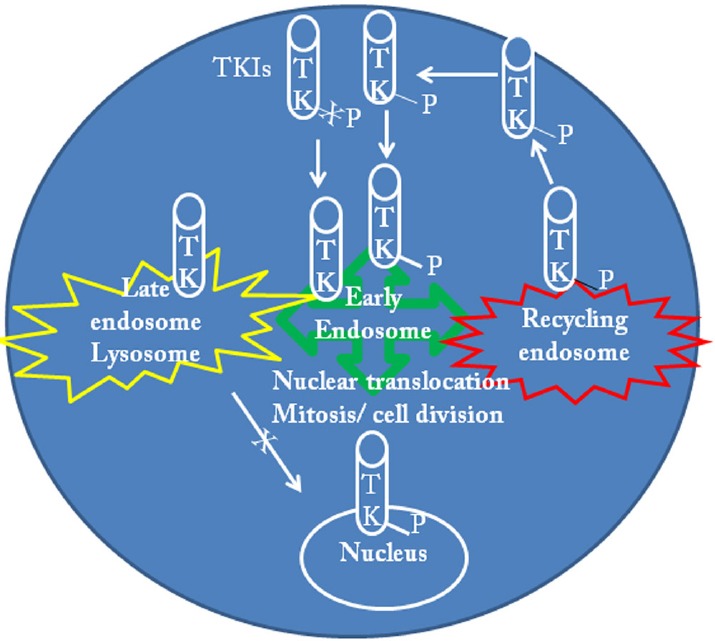
The role of tyrosine kinases (TK) in the modulation of the endosome-lysosome pathways.
Receptor and non-receptor TK are activated via phosphorylation and play a critical role in the endosomal system via translocation of transcription factors that can include mitotic cell division in normal and cancer cells. Networks of early and recycling endosomes modulate cell division and differentiation. Breakpoint Cluster Region-Abelson (BCR-ABL) is constitutively activated in chronic myeloid leukemia causing abnormal mitotic division and differentiation of leukemia and leukemic blast cells. ABL inhibition via de-phosphorylation reduces mitotic cell division and shifts signal transduction pathways away from the nucleus to activate the late endosome-lysosome system and degrade oncegenes. Activation of the lysosomal pathway (autophagy) can also degrade essential cytosolic compartments (i.e., mitochondria) and lead to apoptosis. In post-mitotic neurons, where ABL and TKs are activated autophagy is inhibited. Inhibition of TKs activates autophagy and leads to degradation of toxic cytosolic proteins. Pulsatile or ON/OFF autophagy can lead to protein clearance without degradation of cytosolic compartments, avoiding self-cannibalization. Manipulation of autophagy via TK inhibition provides a potentially common drug target for both cancer and neurodegeneration.
