FIGURE 6.
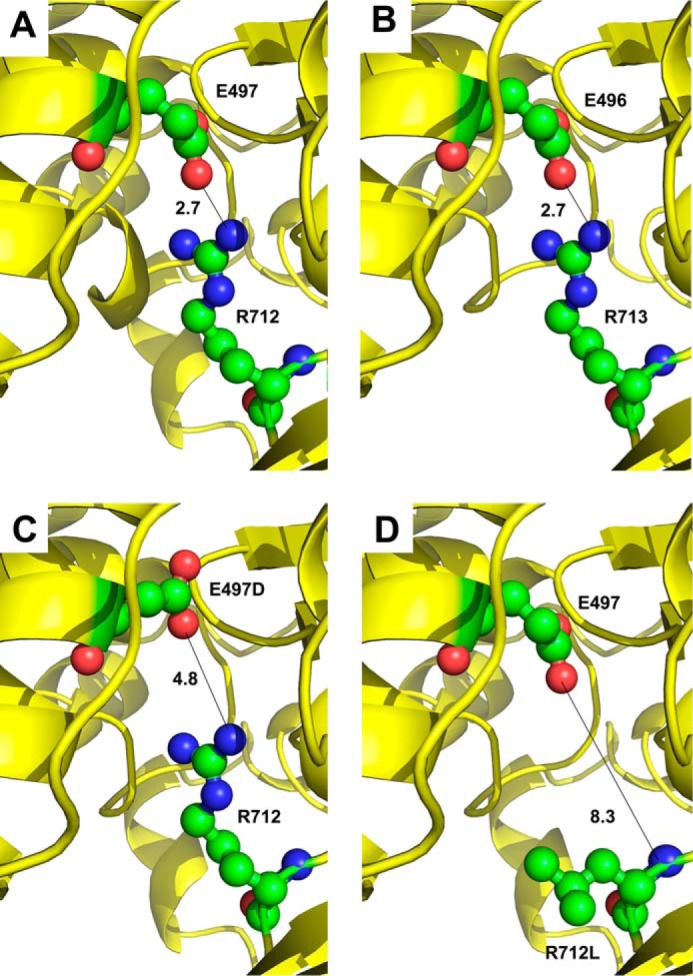
Mapping amino acid residues on the human β-cardiac myosin backbone. A, crystal structure of human β-cardiac myosin (PDB code 4DB1) in an intermediate conformation between the pre-power stroke and actin-detached post-power stroke states displays a salt bridge of 2.7 Å between human residues Glu-497 and Arg-712. B, mapping the Drosophila myosin sequence onto human β-cardiac myosin also yields a salt bridge of 2.7 Å between modeled Drosophila residues Glu-496 and Arg-713. A short N-terminal helix is absent in this model compared with panel A. C, HCM mutation E497D disrupts salt bridge formation with Arg-712 in human β-cardiac myosin, resulting in a distance of 4.8 Å between the charged atoms. D, HCM mutation R712L disrupts salt bridge formation with Glu-497 in human β-cardiac myosin, resulting in a distance of 8.3 Å between the nearest residues.
