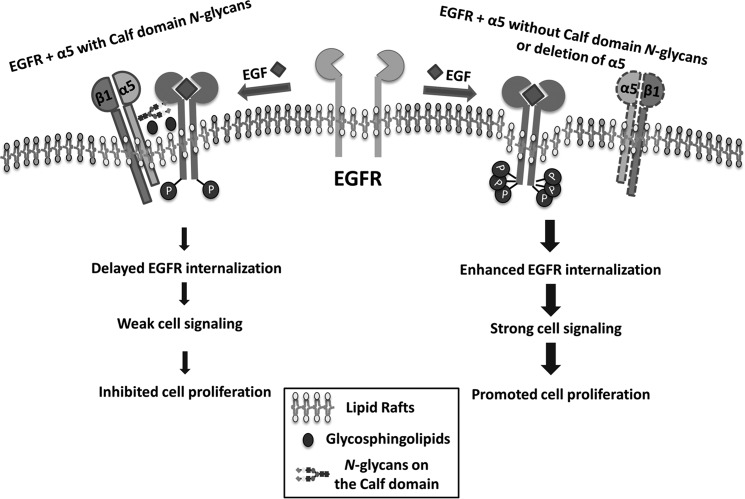
FIGURE 8.
Proposed molecular mechanism for the regulation of EGFR cellular signaling by the N-glycosylations of integrin α5. In the integrin α5 with N-glycosylation on its Calf domain cells (left), integrin α5β1 forms a complex with EGFR as well as glycosphingolipids in the lipid raft, which may restrict the EGFR internalization and the related signaling upon EGF stimulation, resulting in an inhibition of cell proliferation. However, in the integrin α5-deficient or mutant (deletion of N-glycosylation on its Calf domain) cells (right), the majority of EGFR is located in lipid rafts without integrin α5, leading to a rapid activation and internalization of EGFR upon EGF stimulation. The thick arrow lines indicate a rapid EGF response and strong signal transduction, whereas the thinner arrow lines indicate that these events are inhibited.