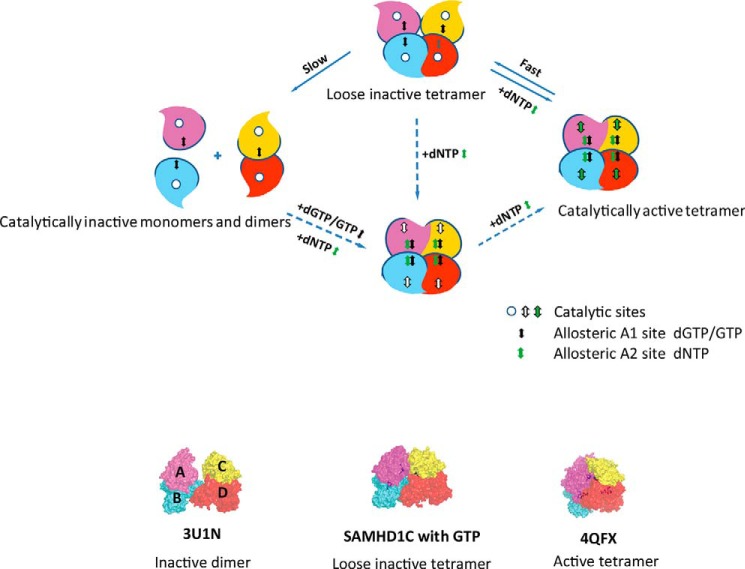
FIGURE 7.
Ordered pathway for activation of SAMHD1. Shown is a model of the dGTP/GTP-dependent allosteric regulation of SAMHD1 catalysis. Subunits of SAMHD1 are labeled A, B, C, and D, and the catalytically inactive monomers and dimers are shown with empty substrate-binding pockets. dGTP/GTP+dNTP could induce an active tetrameric formation of SAMHD1 to hydrolyze the substrates. After the reaction, the tetramer form of SAMHD1 would not immediately dissociate to inactive dimer or monomer states, but instead would prefer to stay in a loose inactive tetramer form that would immediately turn into an active tetramer state in response to the addition of dNTP. The dashed arrows indicate that a form might exist in the pathway. The loose inactive tetramer form containing four GTPs may exist under physiological conditions, and the GTP that might exist in subunit D was shown in gray. Bottom, surface representation of the 3U1N inactive dimer structure, our new loose tetrameric structure, and the 4QFX active tetramer structure. Subunits are in the same color scheme as in Fig. 2.