FIGURE 3.
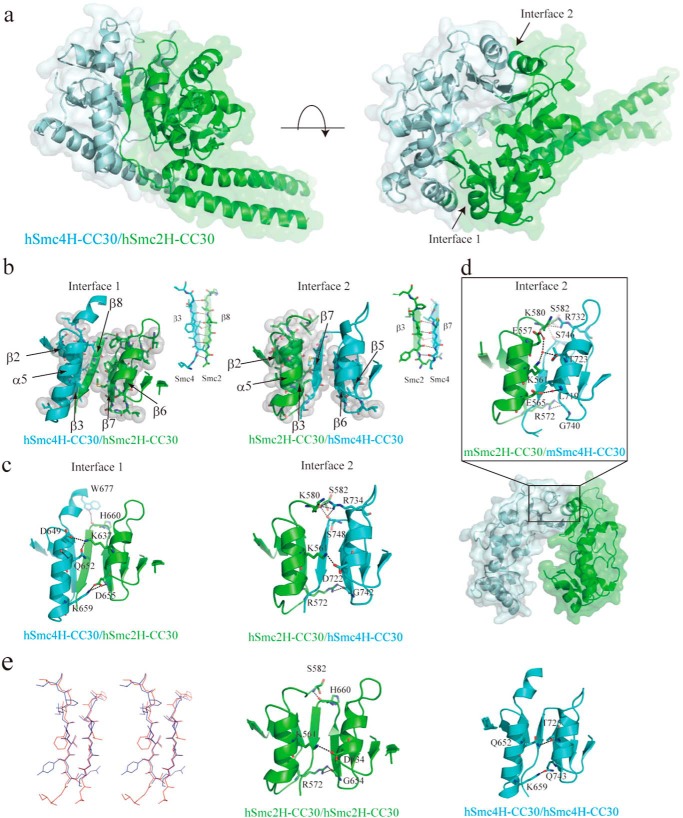
Structural characterization of human condensin Smc hinge-hinge interactions. a, overall structure of hSmc2–4 hinge with middle coiled coils (hSmc2H-CC30/hSmc4H-CC30). The hSmc2H-CC30 and hSmc4H-CC30 are colored light green and light blue, respectively. b, two dimerization interfaces (interface 1 and interface 2) between the hSmc2H-CC30 and hSmc2H-CC30 designated by a black arrow in the right panel of a. The hydrophobic interaction at two dimerization interfaces; interface 1 (left panel) and interface 2 (right panel) is shown. Amino acid residues involved in the interactions are indicated as stick and sphere models. The anti-parallel β-sheet interactions, with typically 4–6 intermolecular hydrogen bonds, are also indicated. c, three key intermolecular hydrogen bonding interactions at the top, middle, and bottom regions of the outer surface at each of the dimerization interfaces. d, dimerization interface observed in a mouse Smc2/Smc4 hinge heterodimer crystal (Protein Data Bank code 3L51), corresponding to interface 2 of the hSmc2H-CC30/hSmc4H-CC30 crystal structure. e, stereo view of the structural superposition of two subunit-subunit antiparallel β-sheets of the hSmc2H-CC30/hSmc4H-CC30. The β-sheets formed at interface 1 and 2 are colored red and light blue, respectively. Dimerization interface of hypothetical homodimer models of hSmc2H-CC30/hSmc2H-CC30 (middle panel) and hSmc4H-CC30/hSmc4H-CC30 (right panel) is also shown. The key interacting residues in b–e are presented as a stick model.