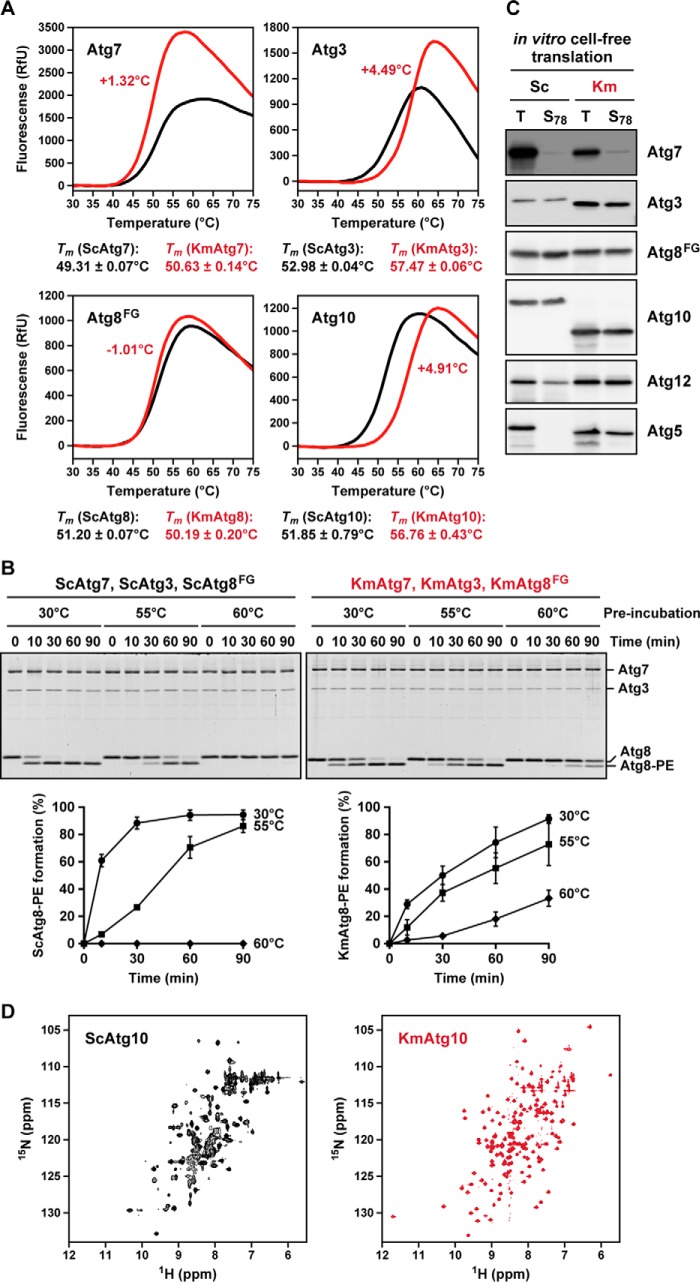
FIGURE 5.
KmAtg proteins are more thermostable and soluble than ScAtg proteins. A, KmAtg7, KmAtg3, and KmAtg10 are more thermostable than ScAtg7, ScAtg3, and ScAtg10, respectively. Recombinant Atg proteins (Atg7, Atg3, Atg8FG, and Atg10) derived from K. marxianus (red) and S. cerevisiae (black) were subjected to DSF analysis. B, KmAtg3 is more thermostable than ScAtg3. Recombinant Atg proteins (0.22 μm Atg7, 0.22 μm Atg3, and 5 μm Atg8FG) derived from S. cerevisiae (left panel) and K. marxianus (right panel) were subjected to in vitro PE-conjugation assay (350 mm PE-containing liposome). Before conjugation reaction, ScAtg3 and KmAtg3 were preincubated at 30, 55, or 60 °C for 90 min, and the conjugation reaction was performed at 30 °C for 10, 30, 60, and 90 min. The samples were subjected to urea-containing SDS-PAGE followed by Coomassie Brilliant Blue staining. The PE-conjugated form of Atg8 (Atg8-PE) was quantitated. Total amounts of Atg8 were defined as 100%. RfU, relative fluorescence unit. C, strep-tagged Atg proteins (Atg7, Atg3, Atg8FG, Atg10, Atg12, and Atg5) derived from K. marxianus and S. cerevisiae were expressed at 37 °C for 4 h by using the PUREfrex cell-free translation kit (GeneFrontier). After the translation, the total reaction mixtures (T) were centrifuged at 78,000 × g for 30 min, and supernatants were prepared (S78). The samples were subjected to immunoblot analysis using anti-Strep antibody. D, NMR spectra of ScAtg10 (left panel) and KmAtg10 (right panel).