FIGURE 1.
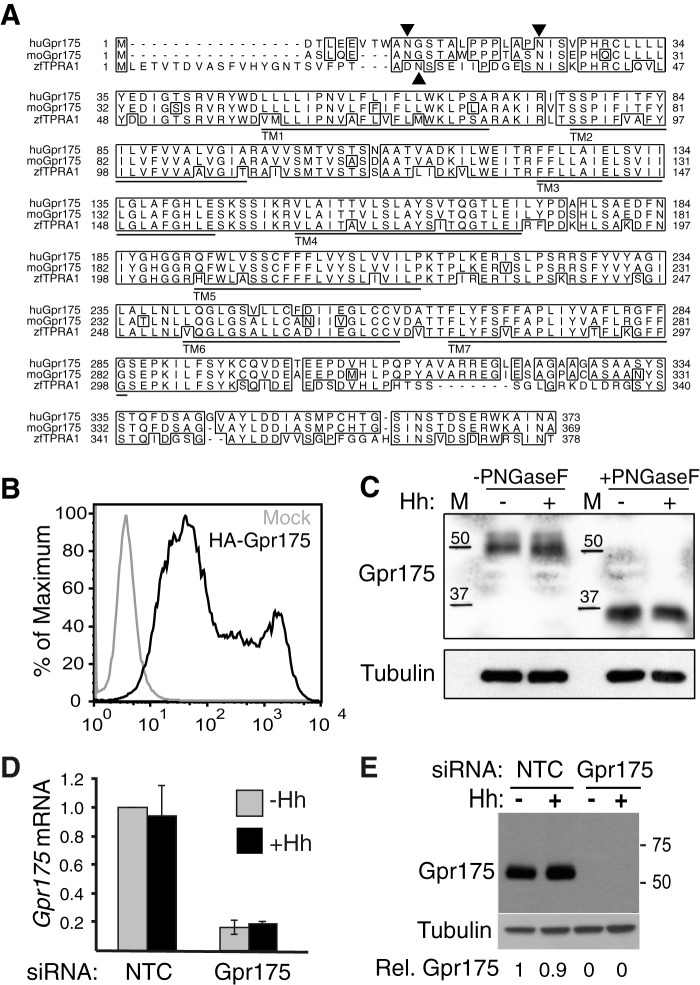
Gpr175 is a conserved glycosylated GPCR-like protein. A, sequence alignment of Gpr175 protein showing 91% identity between human (Q86W33) and mouse (Q99MU1), and 70% identity between human and zebrafish Tpra1 (Q4V8X0). The seven putative transmembrane (TM) domains are underlined, identical residues are boxed, and putative N-glycosylation (NxS) sites are marked with an arrowhead. B, the N terminus of Gpr175 is extracellular, as predicted for a GPCR. Flow cytometry analysis of HEK-293T cells transfected with empty vector (gray) or N terminally tagged HA-Gpr175 (black) for 48 h and labeled with Alexa 488-conjugated anti-HA without permeabilization. C, endogenous murine Gpr175 is N-glycosylated. Western blot of S12 cells treated ±Hh, with lysates treated with PNGase F or buffer alone for 1 h at room temperature, probed with rabbit anti-Gpr175 polyclonal antibody 4241 or with tubulin antibody 1A2 as a loading control. M, molecular mass markers in kDa. D, 80% depletion of Gpr175 mRNA in S12 cells by treatment with siGpr175 with (black) or without Hh (gray) measured by qRT-PCR. Data were normalized to the housekeeping gene mRpl19 mRNA and expressed relative to siNTC −Hh control. Mean ± S.D. of three individual experiments are shown. E, Western blot with rabbit polyclonal anti-Gpr175 showing endogenous Gpr175 protein in S12 cell lysates (40 μg/lane) is apparently completely depleted by siGpr175 (±Hh stimulation) in parallel with an experiment shown in Fig. 2A. Similar results were obtained with an independent anti-GPR175 antibody (6H2, data not shown). Molecular mass markers in kDa are shown on the right. Quantification of Gpr175 relative to tubulin loading control and NTC-Hh is shown under the blot, which is representative of one done three times.