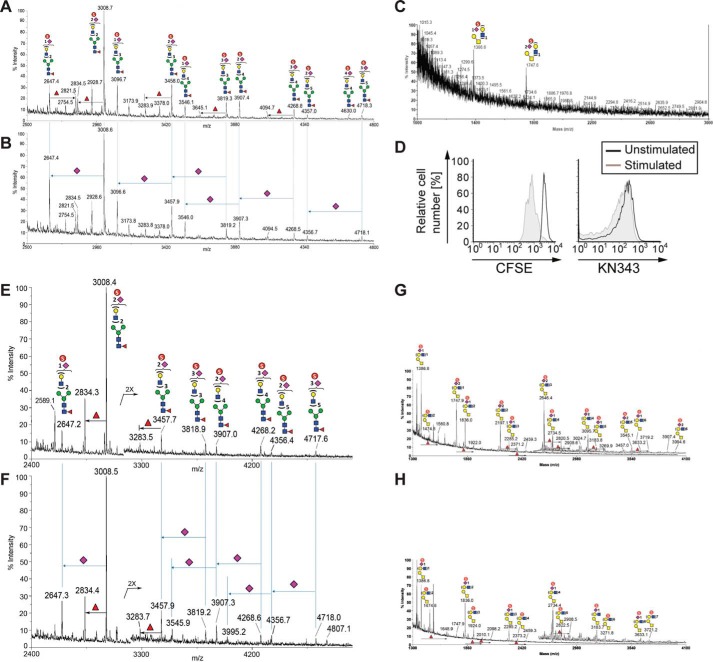
FIGURE 4.
Mass spectrometry analyses reveal that human tonsillar B-cells contain an abundance of sulfated sialylated glycans. A–C, human tonsillar B-cells (200 × 106) were purified by negative selection, and N-glycans from extracted glycoproteins were released by peptide:N-glycosidase F digestion, without (A) or with (B) further digestion by an α2–3-specific sialidase and permethylated, and the enriched sulfated glycan fractions were analyzed by MALDI-MS in negative ion mode. C, analysis of O-glycan analysis of human tonsillar B-cells. D, human peripheral blood B-cells were purified by negative selection, labeled with CFSE, and stimulated with a combination of anti-IgM, anti-IgG, anti-IgA, and CpG2006. After 3 days in culture, stimulated cells were compared with non-stimulated cells for levels of CFSE and KN343 staining. E–H, similar negative ion mode sulfoglycomic profilings were carried out on permethylated N-glycans (E and F) and from stimulated peripheral blood B-cells (G and H) before (E and G) and after (F and H) digestion with a α2–3-specific sialidase. The major [M − H]− sulfated N-glycan and O-glycan signals were assigned by glycosyl compositions fitted in terms of Hex, HexNAc, fucose, and NeuAc, and the most likely structures annotated using the standard schematic symbols recommended by the Consortium for Functional Glycomics, without implying specific linkages and stereochemistry. Structures related by a single NeuAc residue difference are indicated in the mass spectra for sialidase-treated sample.