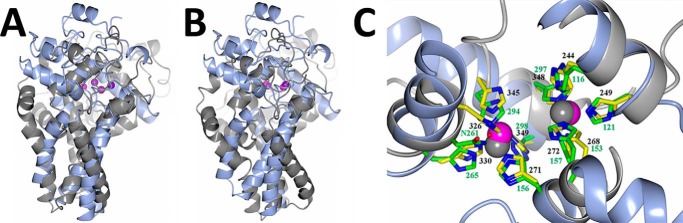
FIGURE 5.
Superposition between scScs7p and SCD1. Schematic rendering of the superposition between scScs7p (light blue) and mouse SCD1 (gray). Although the overall fold is conserved between the two enzymes, there are significant differences between the helices in the transmembrane stalk and the catalytic cap domain, regardless of whether the superposition is carried out based on secondary structural elements (A) or when the zinc atoms and histidine box residues are utilized for the superposition (B). C, dimetal center is the most conserved region between the two enzymes. Histidine side chains from scScs7p are colored with yellow carbons and numbered in black font, and those from mouse SCD1 are colored with green carbons and numbered in green font. Note that SCD1 coordinates the two zinc atoms within the dimetal center with nine histidine residues and Asn-261 via a bridging water molecule (red). Nitrogen atoms are colored dark blue. The zinc atoms from scScs7p are colored magenta, and those from SCD1 are colored gray.