FIGURE 2.
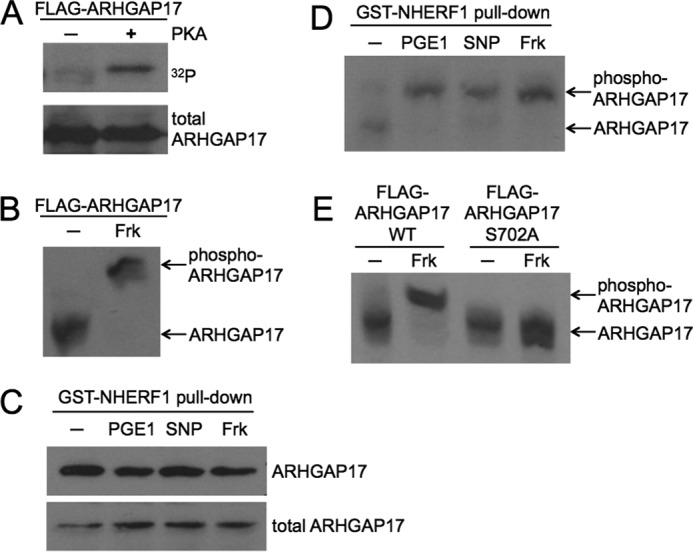
The GTPase-activating protein ARHGAP17 is a target of cyclic nucleotide signaling. A, ARHGAP17 is phosphorylated by PKA in vitro. FLAG-ARHGAP17 was expressed in HEK293T cells, immunoprecipitated using anti-FLAG beads, and incubated with purified catalytic subunit of PKA in the presence of [γ-32P]ATP for 4 min. The reaction was stopped by addition of SDS-sample buffer, subjected to SDS-PAGE and blotting. Radiolabeled ARHGAP17 was detected by autoradiography (upper panel) and total ARHGAP17 levels were determined by Western blotting with anti-FLAG antibody (lower panel). Data are representative of three independent experiments. B, ARHGAP17 is phosphorylated by PKA in intact cells. HEK293T cells expressing FLAG-ARHGAP17 were treated without or with forskolin (Frk, 10 μm, 10 min) to activate endogenous PKA and lysed. Samples were subjected to Zn2+-Phos-tag SDS-PAGE as described under “Experimental Procedures” followed by Western blotting using an anti-FLAG antibody. The shifted, phosphorylated form of ARHGAP17 is indicated. Data are representative of three independent experiments. C, NHERF1 precipitates endogenous ARHGAP17 from platelet lysates. Washed human platelets were incubated with PGE1 (0.5 μm, 1 min), SNP (10 μm, 10 min), or forskolin (10 μm, 15 min). Platelets were lysed and purified GST-NHERF1 was used to pull down endogenous ARHGAP17. Samples were analyzed by Western blotting using anti-ARHGAP17 antibody to determine precipitated (upper panel) and total ARHGAP17 levels (lower panel). Data are representative of two independent experiments. D, endogenous ARHGAP17 is phosphorylated by endogenous PKA and PKG in platelets. Washed human platelets were incubated with PGE1 (0.5 μm, 1 min) or forskolin (10 μm, 15 min) to activate PKA, or with the NO-donor SNP (10 μm, 10 min) to activate PKG, lysed, and GST-NHERF1 pull-down assays were performed to precipitate ARHGAP17 as described in C. Samples were subjected to Zn2+-Phos-tag SDS-PAGE and Western blotting as described in B and endogenous ARHGAP17 was detected by anti-ARHGAP17 antibody. The shifted, phosphorylated form of ARHGAP17 is indicated. Data are representative of three independent experiments. E, Ser-702 is the PKA phosphorylation site on ARHGAP17. HEK293T cells expressing wild type FLAG-ARHGAP17 or mutant FLAG-ARHGAP17-S702A were incubated without or with forskolin (10 μm, 10 min) and lysed. Samples were subjected to Zn2+-Phos-tag SDS-PAGE followed by Western blotting using an anti-FLAG antibody. The shifted, phosphorylated form of ARHGAP17 is indicated. Data are representative of two independent experiments.
