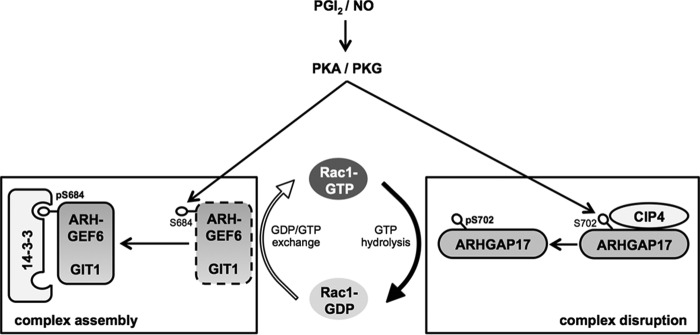
FIGURE 6.
Model of Rac1 inhibition during PKA and PKG activation. Upon platelet activation Rac1-GTP levels rise rapidly and Rac1-GTP is able to interact with its effector molecules to promote downstream signaling. Endothelial NO and PGI2, the two most potent endogenous platelet inhibitors described to date, are able to inhibit agonist induced Rac1-GTP formation. ARHGAP17 is a Rho GTPase-activating protein of Rac1 and is bound to the SH3 domain of CIP4 via its SH3 binding region in resting platelets. Endothelial PGI2 stimulates the activation of PKA and leads to the phosphorylation of Ser-702 in ARHGAP17, which results in the dissociation of the ARHGAP17-CIP4 complex. ARHGEF6 is a Rho guanine nucleotide exchange factor for Rac1 and constitutively bound to GIT1. NO and PGI2 activate PKG and PKA, respectively and both kinases phosphorylate ARHGEF6 on Ser-684 and possibly on Ser-640. Phosphorylation of ARHGEF6 results in the assembly of a GIT1-ARHGEF6–14-3-3 complex. These changes might contribute to PGI2- and NO-mediated Rac1 inhibition.