Background: B7-H3 is an immune inhibitory ligand and an antigen on many solid tumors.
Results: Antibody 8H9 was humanized and affinity-matured, and its epitope was mapped to the FG loop of B7-H3.
Conclusion: hu8H9 antibodies had potent antitumor activity and may modulate immune inhibitory properties of B7-H3.
Significance: Antibodies were developed to target solid tumors and affect immune checkpoint blockade.
Keywords: antibody engineering, cancer therapy, homology modeling, immunotherapy, molecular docking
Abstract
B7-H3 (CD276) is both an inhibitory ligand for natural killer cells and T cells and a tumor antigen that is widely expressed among human solid tumors. Anti-B7-H3 mouse monoclonal antibody 8H9 has been successfully used for radioimmunotherapy for patients with B7-H3(+) tumors. We present the humanization, affinity maturation, and epitope mapping of 8H9 based on structure determination, modeling, and yeast display methods. The crystal structure of ch8H9 Fab fragment was solved to 2.5-Å resolution and used as a template for humanization. By displaying the humanized 8H9 single chain Fv (scFv) on the surface of yeast, the affinity was matured by sequential random mutagenesis and fluorescence-activated cell sorting. Six mutations (three in the complementarity-determining region and three in the framework regions) were identified and incorporated into an affinity-matured humanized 8H9 construct (hu8H9-6m) and an affinity-matured chimeric 8H9 construct (ch8H9-6m). The hu8H9-6m scFv had a 160-fold improvement in affinity (0.9 nm KD) compared with parental hu8H9 scFv (144 nm KD). The IgG formats of ch8H9-6m and hu8H9-6m (nanomolar to subnanomolar KD) had 2–9-fold enhancements in affinity compared with their parental forms, potent in vitro antibody-dependent cell-mediated cytotoxicity (0.1–0.3 μg/ml EC50), and high tumor uptake in mouse xenografts. Based on in silico docking studies and experimental validation, the molecular epitope of 8H9 was determined to be dependent on the FG loop of B7-H3, a region critical to its function in immunologic blockade and unique among anti-B7-H3 antibodies published to date.
Introduction
Removal of negative regulatory pathways for immune cells is rapidly gaining importance in cancer treatment. The first successful example of such an immune approach was the anti-CTLA4 monoclonal antibody (mAb) ipilimumab (1), which became Food and Drug Administration-approved for melanoma. An equally exciting strategy targeted the programmed death (PD)2-1 T cell co-receptor and its ligands B7-H1/PD-L1 and B7-DC/PD-L2, a pathway that maintains an immunosuppressive tumor microenvironment (2). Phase I/II clinical trials of anti-PD-1 (3) or anti-PD-L1 (4) have shown tumor responses in melanoma, non-small cell lung cancer, renal cell carcinoma, and ovarian cancer. Besides waking up T cells, removing inhibitory signals on NK cells could also have clinical potential for both hematological and solid malignancies (5–7). Blocking mAbs (e.g. anti-CD47) has also been effective in unleashing macrophages to phagocytose tumor cells in the absence or presence of mAb (8) both in vitro and in vivo in leukemia/lymphoma (9–13) as well as in solid tumor models (11, 13). Because B7-H3 is inhibitory for both NK cells and T cells, antibodies directed at B7-H3, besides being directly cytotoxic for tumors, may also remove the inhibitory signal to the immune system.
Human B7-H3 (also named as CD276) is a member of the B7/CD28 immunoglobulin superfamily, which provides crucial costimulatory signals that regulate T cell functions in tumor surveillance, infections, and autoimmune diseases (14). B7-H3 was initially identified as a type I transmembrane protein with its extracellular region containing only one V-like and C-like Ig domain (2Ig-B7-H3) (15), similar to all other B7 family members. Subsequently, a second dominantly expressed form of human B7-H3 that contained a tandemly duplicated V-like and C-like Ig domain (4Ig-B7-H3) was found (16, 17). The inhibitory role of B7-H3 was supported by the reports that both 2Ig and 4Ig forms of human B7-H3 inhibited T cell proliferation and cytokine production (16). B7-H3 preferentially down-regulated the TH1-mediated immune response in B7-H3-deficient mice (18), and 4Ig-B7-H3 inhibited NK-mediated lysis of neuroblastoma cells by interacting with a putative inhibitory receptor on the surface of NK cells (19). In more recent studies of patients with prostate cancer, tumor B7-H3 expression was strongly correlated with disease spread at time of surgery, increased risk of clinical cancer recurrence, and cancer-specific death (20, 21). Moreover, tumor B7-H3 expression was correlated with poor patient survival in clear cell renal cell carcinoma, urothelial cell carcinoma (22, 23), ovarian cancer (24), glioblastoma (25), osteosarcoma (26), pancreatic cancer (27), and neuroblastoma (28). A few earlier reports have also implicated a positive immunologic function of B7-H3; e.g. human B7-H3 (2Ig form) promoted T cell activation and IFN-γ production by binding to a putative receptor on activated T cells (15). Furthermore, the antitumor response was enhanced by B7-H3 expression in murine tumor models (29), and B7-H3 positivity was correlated with increased survival in gastric cancer (30) and pancreatic cancer (31). It is possible that B7-H3 has both co-inhibitory and co-stimulatory properties depending on the receptors (32). Based on the mouse B7-H3 crystal structure, the FG loop of the IgV domain was identified to play a critical role in its T cell inhibitory function (33). B7-H3 is widely expressed among solid tumors, including prostate (20, 21), renal cell carcinoma, urothelial cell carcinoma (22, 23), ovarian cancer (24), glioblastoma (25), osteosarcoma (26), neuroblastoma (28), diffuse intrinsic pontine glioma (34), mesothelioma (35), and pancreatic cancer (27).
8H9 is a murine IgG1 mAb that targets B7-H3 (36, 37). By immunostaining, it is broadly reactive with human solid tumors, including embryonal tumors and carcinomas (36). It has shown favorable tumor uptake for both sarcoma and brain tumors in xenograft models (38, 39). When conjugated to cobra venom factor, it induces efficient complement-mediated tumor lysis (40). In its single chain Fv (scFv) format, it targets a potent immunotoxin to sarcoma and glioma in preclinical models (41, 42). As a chimeric antigen receptor, it redirects natural killer cells to kill B7-H3(+)-positive tumor cells (43). In early phase human clinical trials, 131I-8H9 prolongs survival among high risk patients with solid tumors suffering from central nervous system (CNS) metastasis (44–46). It is a promising target for radioimmunotherapy of leptomeningeal metastases (NCT00089245) (46), diffuse intrinsic pontine glioma (NCT01502917) (34), and peritoneal metastases (NCT01099644). The only other antibody in phase I trial is MGA271, a humanized IgG1 mAb (NCT0139114) (47). B7-H3 expression at the mRNA and protein levels is regulated by microRNA miR-29 in tumors and normal tissues (37). When neuroblastoma metastasized to the brain, its miR-29 was down-regulated, which was associated with increased expression of several oncoproteins as well as B7-H3 (48).
Here we describe a strategy to humanize and affinity mature mAb 8H9 based on x-ray crystallography, in silico modeling, and yeast display. We were additionally able to predict and validate the molecular epitope on B7-H3 to be dependent on the FG loop, which may affect the immune modulatory properties of B7-H3.
Experimental Procedures
X-ray Crystallography
Fab fragments of ch8H9 were generated by papain digestion using a standard Fab preparation kit (Pierce). The purified Fab fragment was concentrated to 10 mg/ml in 20 mm HEPES, pH 6.5, and was crystallized in a hanging drop by vapor diffusion at 16 °C against a reservoir containing Hampton Index reagent containing 0.2 m lithium sulfate monohydrate, 0.1 m Bis-Tris, pH 6.5, 25% (w/v) polyethylene glycol 3,350 (Hampton Research, Aliso Viejo, CA). The droplet was formed by mixing 1 μl of protein solution and 1 μl of reservoir solution. Data were collected at the Argonne Advanced Photon Source beamline 24IDE. The Fab structure was solved by molecular replacement using MOLREP (49). The best molecular replacement model was refined using PHENIX (50), and manual fitting was performed with O (51).
Molecular Simulations of ch8H9 Structure
The crystal structure was simulated using CHARMm (Chemistry at Harvard Molecular Mechanics) force fields, and the effect of each point mutation was calculated from the difference between the folding free energy of the mutated structure and the wild type protein. Generalized Born approximation was used to account for the effect of the solvent, and all electrostatic terms were calculated as a sum of coulombic interactions and polar contributions to the solvation energy. A weighted sum of the van der Waals, electrostatic, entropy, and non-polar terms was calculated for each point mutation. All calculations were performed using Discovery Studio (Dassault Systems, San Diego, CA). Docking simulations were generated using ZDOCK (52), and homology modeling of B7-H3 was done using MODELLER (53). Electrostatic surface potentials were generated using DelPhi (54). All images were rendered using Discovery Studio.
Biotinylation of B7-H3 Antigen
The gene of human B7-H3-mouse Fc (mFc) fusion was optimized for CHO cells (Genscript). Using the Bluescript vector, the gene was transfected into DG44 cells and expressed as described previously (55). Biotinylation of B7-H3-mFc was performed using EZ-Link sulfo-NHS-biotin and biotinylation kit (Thermo Scientific) according manufacturer's instructions. The biotinylated protein was subsequently concentrated using a 50,000-molecular weight cutoff Vivaspin centrifuge tube (Sartorius Stedim) and tested for its biotinylation in streptavidin binding ELISA.
Mutagenesis by Error-prone PCR
Error-prone PCR of the entire hu8H9 scFv (V3) gene was performed using Stratagene GeneMorph® II Random Mutagenesis kit according to the instructions of the manufacturer. Reaction products were purified by 1% agarose gel electrophoresis and concentrated by ultrafiltration with water.
Selection of hu8H9 Mutants from the Yeast Libraries
Construction and growth of yeast libraries for affinity maturation were modified from previous publications (56, 57). Before fluorescence-activated cell sorting (FACS), the yeast library (1 × 109 cells) was preincubated with control mouse 3F8 IgG, sequentially panned against 10 μg of B7-H3-mFc-conjugated magnetic beads, and separated with a magnetic stand. For the first yeast library, three FACS selections were performed with 10, 3, and 1 μg/ml biotinylated B7-H3-mFc, respectively. Yeast plasmids were isolated using Zymoprep Yeast Plasmid Miniprep II kit (Zymo Research) according to the manufacturer's instructions and used for templates of the second library construction. The construction and selection of the second library were the same as for the first yeast library. However, four FACS selections were performed in the second yeast library with 1, 0.2, 0.05, and 0.01 μg/ml biotinylated B7-H3-mFc, respectively. Cells from the last selection were spread on SD-CAA (18.2% sorbitol, 2.0% glucose, 0.67% yeast nitrogen base, 0.5% casamino acids, 0.54% disodium phosphate, 0.86% monosodium phosphate, and 1.5% agar) plates. Monoclonal yeast cells were characterized, and isolated antibodies with improved affinity were sequenced.
Expression and Purification of Soluble scFv
scFv fragments were expressed and purified as described previously (56). All recombinant scFv fragments had FLAG and His tags.
Expression and Purification of IgG Constructs
Using a Bluescript vector (Eureka Therapeutics), the heavy and light chain genes of 8H9 constructs were transfected into CHO-S cells and selected with G418 (Invitrogen). 8H9 producer lines were cultured in OptiCHO serum-free medium (Invitrogen), and the mature supernatant was harvested. A protein A affinity column was pre-equilibrated with 25 mm sodium citrate buffer with 0.15 m NaCl, pH 8.2. Bound 8H9 was eluted with 0.1 m citric acid, sodium citrate buffer, pH 3.9, and dialyzed in 25 mm sodium citrate, 150 mm NaCl, pH 8.2.
Affinity Determination by Surface Plasmon Resonance
In brief, B7-H3 antigen (either 2Ig-B7-H3, 4Ig-B7-H3, 2Ig-B7-H3-mFc, or 4Ig-B7-H3-mFc) was immobilized onto the CM5 sensor chip via amine coupling. A reference surface was set up for nonspecific binding and refractive index changes. For analysis of the kinetics of interactions, varying concentrations of scFv fragments (25–800 nm) or IgG (6.25–400 nm) were injected at a flow rate of 30 μl/min using running buffer containing 10 mm HEPES, 150 mm NaCl, 3 mm EDTA, 0.05% Surfactant P-20, pH 7.4. The association and dissociation phase data were fitted simultaneously to a 1:1 model by using BIAevaluation 3.2. The goodness of fits was determined by examining the χ2 relative to Rmax and the standard errors of the calculated ka and kd values. For IgG samples, the apparent ka and kd values may represent a bivalent interaction. All the experiments were done at 25 °C. For epitope determination, WT 2Ig-B7-H3, R127A, and D128A were immobilized onto the chip, and 400 nm ch8H9, MIH42 (Thermo Scientific Pierce), 6A1 (Thermo Scientific Pierce), and clone 185504 (R&D Systems) were injected as described above (contact time, 180 s; dissociate time, 600 s).
Tumor Cell Culture and Antibody-dependent Cell-mediated Cytotoxicity (ADCC) Assay
Neuroblastoma LAN-1 tumor cells were obtained from Children's Hospital of Los Angeles. Cells were cultured in RPMI 1640 medium (Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; Life Technologies) at 37 °C in a 5% CO2-humidified incubator. ADCC assays were performed as described previously (55). Briefly, LAN-1 neuroblastoma tumor cells were radiolabeled with 51Cr, and peripheral blood mononuclear cells from healthy donors were used as effectors at a 25:1 effector-to-target ratio. Samples were incubated for 4 h at 37 °C and 5% CO2, and then the cytotoxicity was measured by 51Cr release.
Biodistribution of Antibody in Xenografted Mice
Female athymic nude mice (6–8 weeks old) were purchased from Harlan Sprague-Dawley, Inc. All procedures were carried out in accordance with the protocols approved by our Institutional Animal Care and Use Committee and institutional guidelines for the proper and humane use of animals in research. Tumor cells (LAN-1) were harvested, and subcutaneously implanted to the flank of the mice (5 × 106 cells/mouse). When the tumor volumes were ∼200 mm3, randomized groups of mice (n = 4–5/group; one radiolabeled antibody preparation/group) were intravenously injected with 50 μCi of 131I-radioiodinated antibody (prepared according to the IODO-GEN method (58, 59) followed by gel filtration purification using commercial prepacked Sephadex G-25 columns into PBS + 1% BSA; specific activity, 1.67–3.81 mCi/mg; 13–30 μg of antibody/dose). The immunoreactivity of each tracer was evaluated using an in vitro cell binding assay using freshly harvested LAN-1. Mice were sacrificed 48 h postinjection, organs were removed, and radioactivity was counted in a gamma counter (PerkinElmer Life Sciences Wallac Wizard 3). These organs included skin, liver, spleen, kidney, adrenal gland, stomach, small intestine, large intestine, femur, muscle, tumor, heart, lung, spine, and brain. Count rates were background- and decay-corrected, converted to activities using a system calibration factor specific for the isotope, normalized to the administered activity, and expressed as percent injected dose/g. Tumor-to-non-tumor ratios of percent injected dose/g were also calculated.
Results
Crystallization of ch8H9
The crystal structure of ch8H9 Fab fragment was determined by molecular replacement using Protein Data Bank code 3D85 and refined to 2.5-Å resolution. Collection and refinement statistics are shown in Table 1. The final model was deposited in the Protein Data Bank (access code 5CMA).
TABLE 1.
X-ray data collection and refinement statistics
Statistics for the highest resolution shell are shown in parentheses. r.m.s., root mean square.
| Wavelength (Å) | 0.9795 |
| Resolution range (Å) | 29–2.495 (2.584–2.495) |
| Space group | C 2 2 21 |
| Unit cell (Å) | 64.576, 207.422, 85.491 |
| Unit cell (°) | 90, 90, 90 |
| Unique reflections | 20,043 (1,957) |
| Completeness (%) | 98.03 (97.12) |
| Mean I/σ(I) | 10.18 (2.30) |
| Wilson B-factor | 55.01 |
| Rwork | 0.2080 (0.3037) |
| Rfree | 0.2606 (0.3421) |
| Number of non-hydrogen atoms | 3,303 |
| Macromolecules | 3,254 |
| Water | 49 |
| Protein residues | 426 |
| r.m.s. (bonds) | 0.003 |
| r.m.s. (angles) | 0.87 |
| Ramachandran favored (%) | 94.0 |
| Ramachandran allowed (%) | 4.6 |
| Ramachandran outliers (%) | 1.4 |
| Average B-factor | 69.70 |
| Macromolecules | 69.90 |
| Solvent | 58.50 |
The ch8H9 Fab structure has immunoglobulin fold domains common to all Fab structures, and the six complementarity-determining region (CDR) loops (H1, H2, H3, L1, L2, and L3) that form the antigen recognition site had well defined electron densities (see Fig. 1). The electrostatic surface potential of the antigen binding site was calculated using DelPhi (54) (Fig. 1C). The center of the binding site has a large area of positively charged surface area dominated by the L3 and H3 loops. Two pockets of negatively charged surface area (at the H2 and L1 loop regions) flank the central region, indicating that the antigen recognized by 8H9 has a mixed surface charge distribution.
FIGURE 1.
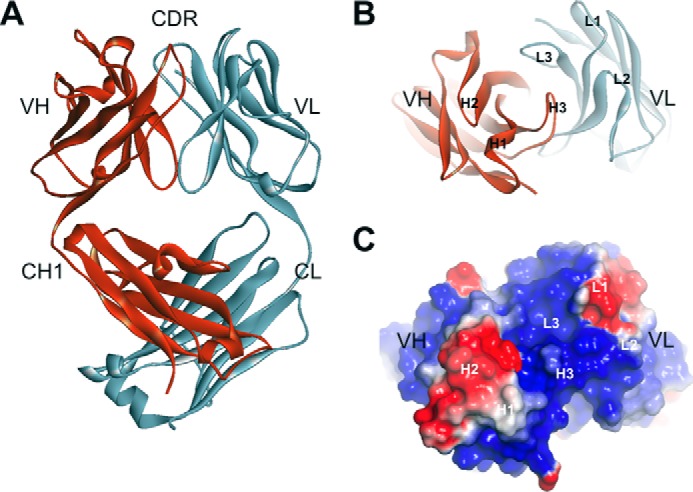
Ribbon diagram of the crystal structure of ch8H9 Fab. A, side view of Fab showing individual Ig domains and CDR. B, top down view on antigen binding site showing the orientation of the 6 CDR loops. C, electrostatic surface potential of antigen binding site rendered from negatively charged (red) to positively charged (blue) in a range from −1 to +1 kT/e.
Humanization of 8H9
The m8H9/ch8H9 antibody was humanized by CDR grafting methods where the CDR loops of the heavy and light chains of m8H9 were grafted onto human IgG1 frameworks based on their homology to human germline sequences IGHV1–46 for the heavy chain and IGKV-7 for the light chain. The framework sequence was then optimized by in silico mutational analysis of the ch8H9 structure based on CHARMm (Chemistry at Harvard Molecular Mechanics) force fields where the effect of each humanizing mutation was calculated from the difference between the folding free energy of the mutated structure and the wild type protein, a method previously described for our humanization of the anti-GD2 mAb 3F8 (60). The resulting hu8H9 (see Table 2) was then used as a template for affinity maturation.
TABLE 2.
Sequence of m8H9/ch8H9 and hu8H9 variable domains with CDR loops underlined
| m8H9/ch8H9 VL | DIVMTQSPATLSVTPGDRVSLSCRASQSISDYLHWYQQKSHESPRLLIKYASQSISGIPSRFSGSGSGSDFTLSINSVEPEDVGVYYCQNGHSFPLTFGAGTKLELKR |
| hu8H9 VL | EIVMTQSPATLSVSPGERVSLSCRASQSISDYLHWYQQKSHESPRLLIKYASQSISGIPARFSGSGSGSEFTLTINSVEPEDVGVYYCQNGHSFPLTFGQGTKLELKR |
| m8H9/ch8H9 VH | QVQLQQSGAELVKPGASVKLSCKASGYTFTNYDINWVRQRPEQGLEWIGWIFPGDGSTQYNEKFKGKATLTTDTSSSTAYMQLSRLTSEDSAVYFCARQTTATWFAYWGQGTLVTVSA |
| hu8H9 VH | QVQLVQSGAEVVKPGASVKLSCKASGYTFTNYDINWVRQRPEQGLEWIGWIFPGDGSTQYNEKFKGKATLTTDTSTSTAYMELSSLRSEDTAVYFCARQTTATWFAYWGQGTLVTVSS |
Affinity Maturation by Yeast Display
A biotinylated B7-H3 construct was used for the selection strategy. For this construct, 4Ig-B7-H3 fused to mouse Fc was expressed in DG44 cells. For biotinylation, NHS-activated biotin was used to react efficiently with primary amino groups (-NH2) in the side chain of lysine residues and the N terminus of each polypeptide. Excess non-reacted biotin-NHS was removed by size exclusion using ultrafiltration columns. The biotinylation of B7-H3-mFc antigen was validated by HRP-conjugated streptavidin using ELISA, and the detection of mouse/human chimeric 8H9 IgG confirmed that biotinylated B7-H3-mFc retained specific binding to 8H9 antibody (data not shown).
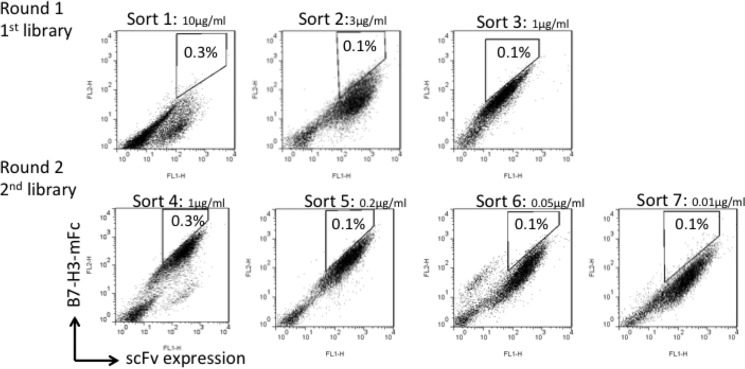
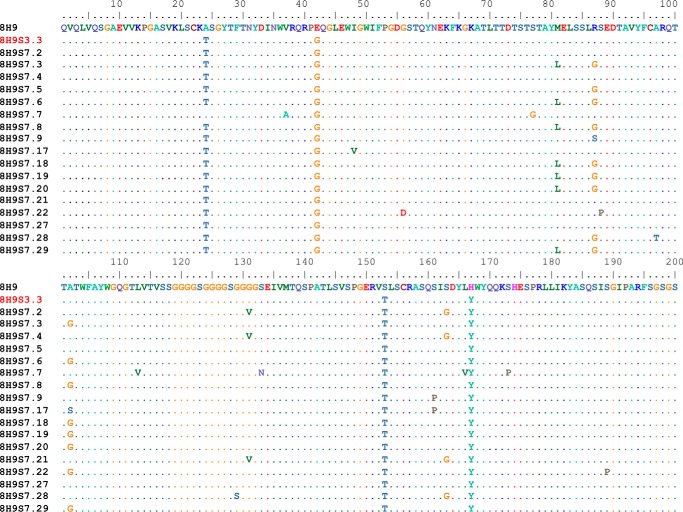
To increase the affinity of hu8H9, it was converted to a scFv (VH-VL format) and randomly mutagenized by error-prone PCR. Previously, we successfully used yeast display for further maturation of antibodies because it allows fine discrimination between mutants by flow cytometry (57, 61). A mutant yeast library of relatively large (up to 108) size was generated and subjected first to a preselection by using biotinylated B7-H3-mFc-conjugated magnetic beads, allowing elimination of yeast cells that did not express antibodies or bound weakly to antigen. The mutant library was then sorted several times by FACS for binding to B7-H3 with stringent conditions (Fig. 2). The sorted scFv fragments were further mutated by error-prone PCR of the entire gene to yield a new sublibrary. The process of sorting and mutagenesis was then cyclically repeated. The highest binding clones to B7-H3 were obtained from the final round of maturation. The Sort 7 yeast showed significantly stronger binding than yeast-displayed parental hu8H9 when they were stained by B7-H3-mFc antigen. The results indicated that the enriched yeast mutants markedly increased the binding affinity. The highest affinity clones from the final round of maturation were identified. Sequence data of strong binders were grouped by cluster analysis, and five repeatedly selected scFv fragments (S7.2, S7.17, S7.22, S7.28, and S7.29) were identified by sequence analysis (Fig. 3).
FIGURE 2.
FACS utilized for the affinity maturation of hu8H9 scFv.
FIGURE 3.
Sequences from Sort 7 yeast cells. Sequence data of strong binders were grouped by cluster analysis, and five repeatedly selected scFv fragments (S7.2, S7.17, S7.22, S7.28, and S7.29) were identified by sequence analysis. S3.3 is the clone from Sort 3. The parental sequence listed at 8H9 is that of hu8H9.
Binding Properties of Selected scFv Mutants
With 4Ig-B7-H3 antigen coated onto CM5 chips, KD values of antibody binding were compared by surface plasmon resonance using Biacore T-100. A summary for KD values of all selected mutants, including hu8H9 (parental) and S3.3, can be found in Table 3. All scFv fragments from Sort 7 respectively bound to 4IgB7-H3 with dissociation constants (KD) of 2, 6, 1, 277, and 3 nm compared with a KD of 144 nm for parental hu8H9 and a KD of 10 nm for S3.3. Among them, scFv fragments S7.17, S7.22, and S7.29 exhibited the highest binding affinity by achieving more than a 50-fold affinity improvement. Clone S7.22 in particular resulted in over a 100-fold enhancement compared with the parental hu8H9 scFv sequence.
TABLE 3.
Binding kinetics of recombinantly expressed scFv against 4I-gB7-H3
Error values are shown as S.E., and Rmax represents the maximum response units (RU) measured for the highest concentration of each sample.
| scFv construct | ka | kd | KD | Rmax | χ2 |
|---|---|---|---|---|---|
| s−1 m−1 | s−1 | nm | RU | RU2 | |
| hu8H9 (parental) | 6.379 ± 0.084 × 103 | 9.185 ± 0.010 × 10−4 | 144.0 | 220.1 | 1.09 |
| S3.3 | 2.481 ± 0.008 × 104 | 2.443 ± 0.007 × 10−4 | 9.85 | 211.0 | 3.05 |
| S7.2 | 2.543 ± 0.007 × 104 | 1.432 ± 0.006 × 10−4 | 5.6 | 210.2 | 2.77 |
| S7.17 | 5.097 ± 0.007 × 104 | 1.134 ± 0.004 × 10−4 | 2.2 | 249.6 | 2.83 |
| S7.22 | 8.614 ± 0.015 × 104 | 9.989 ± 0.061 × 10−5 | 1.2 | 267.8 | 7.77 |
| S7.28 | 4.751 ± 0.100 × 103 | 1.318 ± 0.002 × 10−3 | 277.4 | 248.3 | 1.55 |
| S7.29 | 5.593 ± 0.011 × 104 | 1.519 ± 0.006 × 10−4 | 2.7 | 244.2 | 5.75 |
| hu8H9-6m | 1.035 ± 0.002 × 105 | 9.525 ± 0.066 × 10−5 | 0.92 | 273.2 | 10.2 |
Determination of Key Affinity-enhancing Mutations
Table 4 lists the six mutations identified from affinity maturation sorted sequences (LC, S20T; LC, H34Y; HC, A24T; HC, E42G; HC, G56D; and HC, A102G). Four of the six mutations (LC, S20T; LC, H34Y; HC, A24T; and HC, E42G) were derived from the third round of sorting (clone S3.3) and subsequently present in most of the clones from Sort 7. The two additional mutations (HC, G56D and HC, A102G) were derived from the highest affinity clone S7.22. Three of the six mutations were in CDR regions (LC, H34Y; HC, G56D; and HC, A102G). Two of the six mutations (LC, S20T and HC, E42G) were found in the human germ line templates used for humanization, thereby increasing humanness.
TABLE 4.
Mutations identified from yeast display affinity maturation
| Mutation | Location |
|---|---|
| LC, S20T | Framework |
| LC, H34Y | CDR L1 |
| HC, A24T | Framework |
| HC, E42G | Framework |
| HC, G56D | CDR H2 |
| HC, A102G | CDR H3 |
Affinity-enhanced and Humanized Constructs
The six affinity maturation mutations (LC, S20T; LC, H34Y; HC, A24T; HC, E42G; HC, G56D; and HC, A102G) were incorporated into the hu8H9 sequence to generate hu8H9-6m scFv and IgG1 constructs. The binding kinetics of the scFv constructs (Table 3) for the parental hu8H9 (144 nm KD) and the final hu8H9-6m (0.9 nm KD) showed ∼160-fold enhancement in affinity for the affinity-matured construct.
The binding kinetics for IgG constructs (m8H9, ch8H9, hu8H9, and hu8H9-6m) to both 4Ig-B7-H3 and 2Ig-B7-H3 are shown in Table 5. Both the m8H9 and ch8H9 antibodies bound to the 2Ig-B7-H3 with higher affinity than 4Ig-B7-H3, indicating that the binding epitope may be more fully exposed in the 2Ig-B7-H3 format. The hu8H9 IgG (33 nm KD for 4Ig-B73H3 and 45 nm KD for 2Ig-B7-H3) had partial loss of affinity compared with the ch8H9 (7.7 nm KD for 4Ig-B73H3 and 2.7 nm KD for 2Ig-B7-H3). The affinity-matured hu8H9-6m had a 2.5–9-fold enhancement in affinity (13 nm KD for 4Ig-B73H3 and 5.0 nm KD for 2Ig-B7-H3) compared with the parental hu8H9. Because the hu8H9-6m did not have higher affinity that ch8H9, the six affinity maturation mutations (LC, S20T; LC, H34Y; HC, A24T; HC, E42G; HC, G56D; and HC, A102G) were also incorporated into ch8H9 IgG to generate ch8H9-6m. The binding kinetics of ch8H9-6m are reported in Table 3 (3.7 nm KD for 4Ig-B7-H3 and 0.6 nm KD for 2Ig-B7-H3) showing a 2–4-fold enhancement in binding relative to ch8H9.
TABLE 5.
Binding kinetics of anti-B7-H3 IgG constructs
Error values are shown as S.E., and Rmax represents the maximum response units (RU) measured for the highest concentration of each sample.
| Antibodies | 4Ig-B7-H3 |
2Ig-B7-H3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ka | kd | KD | Rmax | χ2 | ka | kd | KD | Rmax | χ2 | |
| s−1m−1 | s−1 | nm | RU | RU2 | s−1m−1 | s−1 | nm | RU | RU2 | |
| m8H9 | 1.846 ± 0.006 × 104 | 1.650 ± 0.002 × 10−4 | 8.9 | 87.34 | 0.153 | 2.776 ± 0.011 × 104 | 2.244 ± 0.019 × 10−5 | 0.81 | 76.5 | 0.150 |
| ch8H9 | 2.834 ± 0.030 × 104 | 2.180 ± 0.010 × 10−4 | 7.7 | 66.49 | 2.69 | 3.210 ± 0.025 × 104 | 8.729 ± 0.046 × 10−5 | 2.7 | 68.51 | 0.724 |
| ch8H9-6m | 3.349 ± 0.014 × 104 | 1.229 ± 0.002 × 10−4 | 3.7 | 107.1 | 0.550 | 6.890 ± 0.018 × 104 | 4.254 ± 0.026 × 10−5 | 0.62 | 96.84 | 0.844 |
| hu8H9 | 2.046 ± 0.052 × 104 | 6.770 ± 0.023 × 10−4 | 33 | 41.55 | 0.472 | 1.347 ± 0.020 × 104 | 6.069 ± 0.009 × 10−4 | 45 | 57.89 | 0.117 |
| hu8H9-6m | 2.342 ± 0.020 × 104 | 2.979 ± 0.005 × 10−4 | 13 | 82.60 | 0.572 | 3.394 ± 0.011 × 104 | 1.711 ± 0.002 × 10−4 | 5.0 | 80.61 | 0.211 |
In Vitro Tumor Killing Properties of 8H9 Antibodies
We then tested in vitro tumor killing ADCC properties of the five IgG antibody constructs (m8H9, ch8H9, ch8H9-6m, hu8H9, and hu8H9-6m) using neuroblastoma LAN-1 tumor cells as targets and human peripheral blood mononuclear cells as effector cells. Cytotoxicity was measured by chromium-51 release, and the results are shown in Fig. 4 and Table 6. m8H9 had no ADCC function with human peripheral blood mononuclear cells, typical for the murine IgG1 isotype. The ch8H9 and hu8H9 constructs exhibited potent ADCC (1.4 and 0.7 μg/ml EC50, respectively). The ch8H9-6m (0.1 μg/ml EC50) had a 14-fold improvement in cytotoxicity over ch8H9, and hu8H9-6m (0.3 μg/ml EC50) had a 2-fold enhancement in killing over hu8H9 and a 5-fold enhancement in killing over ch8H9. We postulate that the observed effects were mediated by CD16(+) NK cell engagement and not by immune checkpoint blockade, which would not have been easily observed during the 4-h time course of the assay.
FIGURE 4.
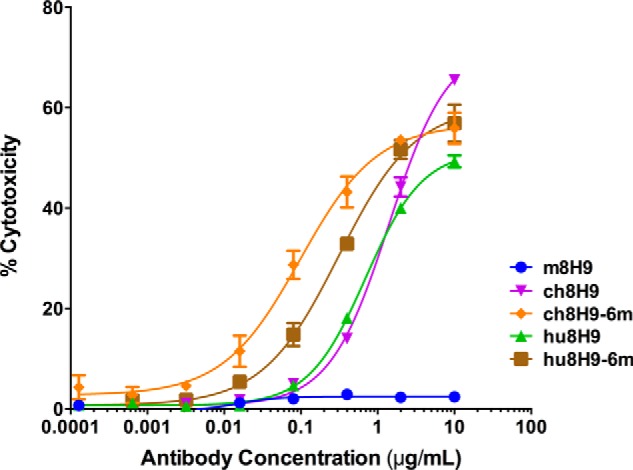
In vitro antibody-dependent cell-mediated cytotoxicity of 8H9 mAbs against B7-H3(+) neuroblastoma LAN-1 cells using human peripheral blood mononuclear cells as effector cells. Cytotoxicity was measured by chromium-51release. Error bars represent S.D.
TABLE 6.
In vitro ADCC assay of anti-B7-H3 IgG constructs
| Antibodies | EC50 | -Fold change in EC50 relative to ch8H9 |
|---|---|---|
| μg/ml | ||
| m8H9 | No killing | |
| ch8H9 | 1.40 ± 0.08 | |
| ch8H9-6m | 0.10 ± 0.01 | 14 |
| hu8H9 | 0.70 ± 0.03 | 2 |
| hu8H9-6m | 0.31 ± 0.03 | 5 |
In Vivo Targeting of Human Neuroblastoma Xenografts
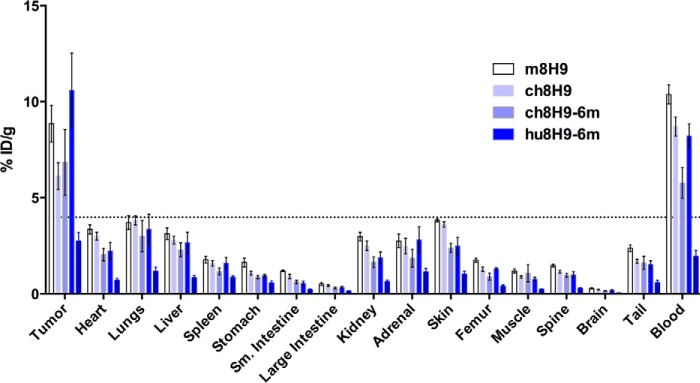
We then determined the in vivo biodistribution of our lead preclinical candidates hu8H9-6m and ch8H9-6m compared with m8H9 and ch8H9 using mouse xenografts. All four antibodies were radiolabeled with 131I, and their in vitro immunoreactivity against LAN-1 cells was determined (m8H9, 69%; ch8H9, 75%; ch8H9-6m, 73%; and hu8H9-6m, 54%). The in vivo biodistributions of each antibody at 48 h postinjection were analyzed using mice bearing subcutaneous neuroblastoma LAN-1 xenografts (Fig. 5 and Table 7). Tumor uptake as measured by percent injected dose/g was 8.9% for m8H9, 6.1% for ch8H9, 6.8% for ch8H9-6m, and 10.6% for hu8H9-6m). Tumor-to-normal tissue ratios are shown in Table 8. All four antibodies had comparable tumor-to-non-tumor ratios for most tissues. hu8H9 had the highest tumor uptake, which was statistically higher than ch8H9 (p < 0.05), and a higher tumor-to-blood ratio than ch8H9 (1.26 compared with 0.72, p < 0.05). ch8H9-6m had a slightly lower tumor uptake but comparable tumor-to-blood ratio.
FIGURE 5.
Biodistribution of 131I-labeled 8H9 mAbs injected into athymic nude mice xenografted with subcutaneous neuroblastoma LAN-1 tumors. ID, injected dose; Sm., small.
TABLE 7.
Biodistribution of 131I-labeled 8H9 antibodies to LAN-1 xenografts in mice
ID, injected dose.
| Organ | % ID/g |
|||||||
|---|---|---|---|---|---|---|---|---|
| m8H9 (n = 5) |
ch8H9 (n = 5) |
ch8H9-6m (n = 4) |
hu8H9-6m (n = 4) |
|||||
| Average | S.E. | Average | S.E. | Average | S.E. | Average | S.E. | |
| Tumor | 8.85 | 0.96 | 6.13 | 0.69 | 6.85 | 1.71 | 10.59 | 1.95 |
| Heart | 3.36 | 0.24 | 3.01 | 0.21 | 2.05 | 0.33 | 2.25 | 0.45 |
| Lungs | 3.72 | 0.36 | 3.83 | 0.23 | 3.02 | 0.80 | 3.37 | 0.78 |
| Liver | 3.14 | 0.31 | 2.81 | 0.20 | 2.30 | 0.37 | 2.67 | 0.55 |
| Spleen | 1.78 | 0.17 | 1.60 | 0.15 | 1.18 | 0.18 | 1.61 | 0.30 |
| Stomach | 1.64 | 0.23 | 1.09 | 0.11 | 0.89 | 0.09 | 0.95 | 0.07 |
| Small intestine | 1.21 | 0.04 | 0.92 | 0.11 | 0.63 | 0.09 | 0.55 | 0.11 |
| Large intestine | 0.52 | 0.08 | 0.45 | 0.05 | 0.32 | 0.05 | 0.33 | 0.06 |
| Kidney | 2.99 | 0.22 | 2.52 | 0.25 | 1.65 | 0.28 | 1.89 | 0.31 |
| Adrenal gland | 2.76 | 0.36 | 2.49 | 0.41 | 1.86 | 0.46 | 2.82 | 0.68 |
| Skin | 3.83 | 0.08 | 3.62 | 0.14 | 2.39 | 0.24 | 2.50 | 0.45 |
| Femur | 1.76 | 0.10 | 1.28 | 0.11 | 0.90 | 0.18 | 1.32 | 0.06 |
| Muscle | 1.20 | 0.11 | 0.88 | 0.06 | 1.08 | 0.45 | 0.77 | 0.11 |
| Spine | 1.47 | 0.07 | 1.15 | 0.07 | 0.98 | 0.09 | 1.01 | 0.15 |
| Brain | 0.29 | 0.03 | 0.22 | 0.02 | 0.15 | 0.02 | 0.18 | 0.03 |
| Tail | 2.38 | 0.17 | 1.70 | 0.10 | 1.63 | 0.34 | 1.54 | 0.19 |
| Blood | 10.38 | 0.49 | 8.70 | 0.50 | 5.77 | 0.79 | 8.21 | 0.63 |
TABLE 8.
Tumor-to-non-tumor uptake ratios of 131I-labeled 8H9 antibodies in LAN-1-xenografted mice
| Organ | Tumor-to-non-tumor ratio |
|||||||
|---|---|---|---|---|---|---|---|---|
| m8H9 (n = 5) |
ch8H9 (n = 5) |
ch8H9-6m (n = 4) |
hu8H9-6m (n = 4) |
|||||
| Average | S.E. | Average | S.E. | Average | S.E. | Average | S.E. | |
| Tumor | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 |
| Heart | 2.67 | 0.31 | 2.09 | 0.28 | 4.09 | 1.62 | 4.98 | 0.66 |
| Lungs | 2.52 | 0.43 | 1.64 | 0.22 | 2.79 | 0.98 | 3.46 | 0.58 |
| Liver | 2.90 | 0.32 | 2.29 | 0.41 | 3.39 | 1.24 | 4.30 | 0.69 |
| Spleen | 5.18 | 0.74 | 4.03 | 0.67 | 6.96 | 2.75 | 6.67 | 0.54 |
| Stomach | 6.40 | 1.98 | 5.77 | 0.71 | 7.89 | 1.99 | 11.05 | 1.59 |
| Small intestine | 7.29 | 0.63 | 7.11 | 1.05 | 13.03 | 5.04 | 20.50 | 3.13 |
| Large intestine | 22.37 | 8.90 | 14.50 | 2.23 | 22.75 | 6.29 | 32.86 | 3.47 |
| Kidney | 3.02 | 0.38 | 2.64 | 0.54 | 4.53 | 1.49 | 5.64 | 0.47 |
| Adrenal gland | 3.31 | 0.33 | 2.92 | 0.75 | 3.88 | 0.74 | 3.94 | 0.37 |
| Skin | 2.31 | 0.24 | 1.73 | 0.26 | 2.92 | 0.76 | 4.42 | 0.55 |
| Femur | 5.04 | 0.48 | 5.12 | 1.06 | 7.96 | 1.63 | 7.96 | 1.31 |
| Muscle | 7.46 | 0.58 | 7.00 | 0.72 | 8.27 | 2.93 | 15.17 | 3.74 |
| Spine | 6.04 | 0.61 | 5.49 | 0.82 | 7.01 | 1.67 | 10.54 | 0.87 |
| Brain | 32.65 | 6.03 | 30.60 | 6.62 | 47.54 | 12.77 | 63.35 | 10.18 |
| Tail | 3.80 | 0.49 | 3.67 | 0.47 | 4.74 | 1.35 | 6.77 | 0.59 |
| Blood | 0.85 | 0.06 | 0.72 | 0.10 | 1.32 | 0.46 | 1.26 | 0.16 |
Epitope Determination
To fully understand how mAb 8H9 recognized the B7-H3 target, in silico methods were used to predict the exact molecular epitope, which was then verified experimentally. The crystal structure of murine 2Ig-B7-H3 (Protein Data Bank code 4IOK) was used as a template to create a homology model of human 2Ig-B7-H3 using MODELLER (53). The human 2Ig-B7-H3 was then used for docking the ch8H9 Fab crystal structure using ZDOCK (52). The top docked model (Fig. 6A) predicted that mAb 8H9 binds the IRDF sequence (residues 126–129) of the V domain of the FG loop in human 2Ig-B7-H3. In 4Ig-B7-H3, this sequence is present twice (residues 126–129 of the V1 domain and residues 344–347 of the V2 domain). The predicted interaction energies for each of the 2Ig-B7-H3-interacting residues of the docked complex are shown in Table 9. Arg-127 and Asp-128 had the highest interaction energies (−55.2 and −46.3 kcal/mol, respectively). Fig. 6B shows how Arg-127 was predicted to interact with the negatively charged surface on the 8H9 L1 CDR loop and how Asp-128 was predicted to interact with the positively charged surface area at the H3 CDR loop, indicating a high degree of charge complementarity.
FIGURE 6.
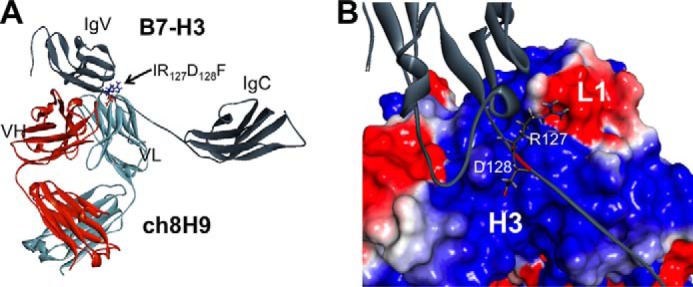
Molecular docking simulation of ch8H9 crystal structure with homology model of human 2Ig-B7-H3. A, ch8H9 is predicted to bind to the IRDF sequence of the extended FG loop. The homology model of human 2Ig-B7-H3 was based on the monomeric unit of the solved crystal structure of mouse B7-H3 (Protein Data Bank code 4I0K). B, close-up view of how Arg-127 was predicted to interact with the negatively charged surface area of the L1 CDR loop of 8H9 and how Asp-128 was predicted to interact with the positively charged surface area of the H3 CDR loop of 8H9.
TABLE 9.
Interaction energies from ch8H9·B7-H3 docked model
Interaction energy was calculated as the sum of van der Waals and electrostatic energy using CHARMm force fields, in the absence of solvation. Arg-127 and Asp-128 are shown in bold to denote the high level of predicted interaction energy.
| 2Ig-B7-H3 residue | Interaction energy |
|---|---|
| kcal/mol | |
| Phe-123 | −3.59 |
| Val-124 | −11.8 |
| Ser-125 | −14.2 |
| Ile-126 | −10.4 |
| Arg-127 | −55.2 |
| Asp-128 | −46.3 |
| Phe-129 | −1.33 |
| Gly-130 | −3.80 |
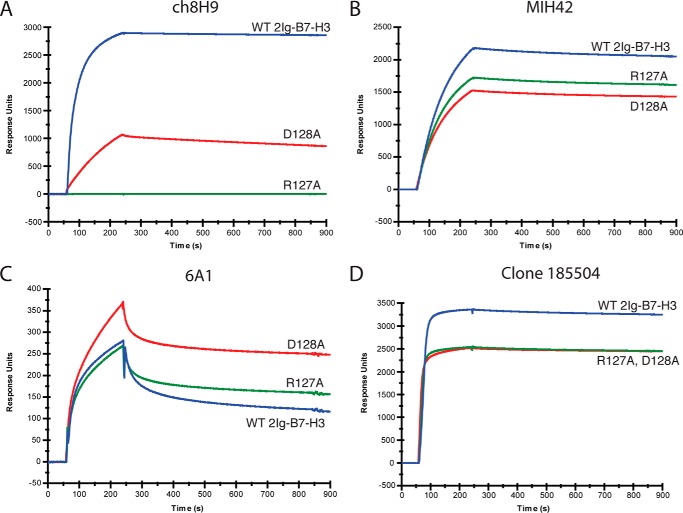
Based on the molecular modeling predictions, R127A and D128A point mutations were experimentally made in two separate 2Ig-B7-H3-Fc constructs, recombinantly expressed, and tested for binding by surface plasmon resonance. Fig. 7 shows the binding kinetics of ch8H9 IgG to the R127A and D128A mutants compared with WT 2Ig-B7-H3 proteins along with the binding of three commercially available anti-B7-H3 mAbs (MIH42, 6A1, and clone 185504). The maximum response units of binding to WT 2Ig-B7-H3-Fc as well as the relative binding of mutants R127A and D128A are shown in Table 10. ch8H9 lost 99.9% of binding to the R127A mutant and 40% binding to the D128A mutant. Antibodies MIH42 and clone 185504 retained ∼70–75% of their respective binding to both R127A and D128A mutants. Antibody 6A1 had weak overall binding to the WT 2Ig-B7-H3 and either the same binding to R127A or elevated binding to the D128A mutants. These binding studies demonstrated that ch8H9 was uniquely specific to the IRDF sequence of B7H3. We also verified that the humanized 8H9 constructs lost binding to the R127A and D128A B7-H3 mutants by surface plasmon resonance (data not shown).
FIGURE 7.
Binding kinetics of anti-B7-H3 antibodies ch8H9, MIH42, 6A1, and clone 185504 to 2Ig-B7-H3-Fc WT, R127A, and D128A by surface plasmon resonance. The relative binding (measured as response units) of the anti-B7-H3 antibodies to the mutants R127A and D128A compared with WT B7-H3 are presented in Table 10.
TABLE 10.
Response units (RU) of binding to WT B7-H3 and mutants R127A and D128A
| Antibody | Maximum RU WT 2Ig-B7-H3 | Relative binding |
|
|---|---|---|---|
| R127A | D128A | ||
| % | |||
| ch8H9 | 2,875 | 0.1 | 60.0 |
| MIH42 | 2,526 | 76.1 | 68.8 |
| 6A1 | 196.1 | 109.1 | 167.5 |
| Clone 185504 | 3,344 | 75.5 | 74.9 |
Discussion
We have developed high affinity chimeric and humanized anti-B7-H3 antibodies based on in silico modeling and yeast display affinity maturation. Both ch8H9-6m and hu8H9-6m achieved nanomolar to subnanomolar KD affinity to B7-H3, showing high potency in vitro ADCC and high in vivo tumor uptake in mouse xenografts. We have additionally used completely in silico molecular docking methods to predict the binding epitope on B7-H3 to be dependent on the IRDF sequence in the FG loop.
The FG loop plays a critical role in receptor recognition in several B7 family member proteins, including PD-L1 (62), PD-L2 (63), B7–1 (64), and B7–2 (65). Although the receptor of B7-H3 is still not definitively known, a structural study of mouse B7-H3 (33) has shown that the FG loop is responsible for the ability of B7-H3 to inhibit T cell proliferation. Mouse B7-H3 only exists as a 2Ig-B7-H3, and the critical sequence responsible for this inhibitory function is IQDF (residues 126–129). We have demonstrated that mAb 8H9 binds to the homologous IRDF (residues 126–129) in human B7-H3 with highest specificity to Arg-127. Additionally, mAb 8H9 and its humanized forms do not bind to the murine B7-H3, which contains Gln-127. Unlike other anti-B7-H3 antibodies tested, only 8H9 can be used to bind the FG loop of B7-H3, which may become relevant for blocking the immune inhibitory functions of human B7-H3. Although we have shown that the binding of mAb 8H9 is FG loop-dependent, we cannot exclude the possibility that other residues may be involved in the binding of this conformational epitope, which is the subject of further investigation. We are also currently testing the ability of mAb 8H9 to inhibit immune checkpoints for cancer immunotherapy.
Unlike any other tumor-associated antigens, B7-H3 is widely expressed among solid tumors and is strongly correlated with recurrence, metastasis, and death. These include prostate cancer, non-small cell lung cancer, pancreatic cancer, colorectal cancer, breast cancer, liver cancer, renal cell carcinoma, urothelial cell carcinoma, ovarian cancer, brain tumors, head and neck cancers, melanoma, soft tissue sarcomas, osteosarcoma, neuroblastoma, diffuse intrinsic pontine glioma, mesothelioma, and desmoplastic small round cell tumor. Such prevalent B7-H3 expression is usually associated with highly aggressive clinical behavior. The discovery of B7-H3 on tumor vasculature has further expanded its utility as a cancer immunotherapy target.
Clinically proven targets for antibody-based therapy share some general properties. Their expression on the cell surface is usually high but low to absent among normal tissues, thereby achieving optimal therapeutic indices. They should be present on tumor stem cells to suppress tumor regrowth. As radioimmunoconjugates they acquire the ability to destroy antigen-negative tumor clones. Alternatively, if the target is on the tumor vasculature, vital supplies to the tumor can be choked off irrespective of whether the antigen is present on all tumor cells. Finally, if the target antigen is critical in immune checkpoints, blocking antibodies have the potential to reverse the negative immune microenvironment. B7-H3 can be exploited for all of these antitumor mechanisms.
We have developed potent chimeric and humanized anti-B7-H3 mAbs that may have clinical potential in Fcγ receptor-dependent immunotherapy, immunoconjugates, and checkpoint blockade. This combination presents a unique and powerful opportunity for maximal antitumor effects directed at a single target for most solid tumors.
Author Contributions
M. A. conceived and executed experiments and co-wrote the manuscript. M. C. conceived and executed experiments. Q. Z. executed experiments related to affinity maturation. Y. G. executed experiments related to x-ray crystallography. S. M. C. conceived and executed experiments related to biodistribution. H.-F. G. executed experiments related to antibody expression and purification. S. M. L. conceived experiments related to biodistribution. N.-K. V. C. supervised all aspects of the investigation and co-wrote the manuscript.
Acknowledgments
We acknowledge Dr. Jian Hu for excellent technical assistance. Experimental support was provided by the Memorial Sloan Kettering Cancer Center X-ray Crystallography Core facility, which is funded by National Institutes of Health Cancer Center Support Grant P30 CA008748. This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by NIGMS, National Institutes of Health Grant P41 GM103403. This research used resources of the Advanced Photon Source, a United States Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357.
M. A., Q. Z., and N.-K. V. C. were named as co-inventors on a patent application for humanized anti-B7-H3 antibodies filed by Memorial Sloan Kettering Cancer Center.
The atomic coordinates and structure factors (code 5CMA) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- PD
- programmed death
- scFv
- single chain Fv
- CDR
- complementarity-determining region
- hu
- humanized
- ch
- chimeric
- NK
- natural killer
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- mFc
- mouse Fc
- NHS
- N-hydroxysuccinimide
- ADCC
- antibody-dependent cell-mediated cytotoxicity
- HC
- heavy chain
- LC
- light chain.
References
- 1.Mellman I., Coukos G., and Dranoff G. (2011) Cancer immunotherapy comes of age. Nature 480, 480–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian S. L., Drake C. G., and Pardoll D. M. (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol.24, 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian S. L., Hodi F. S., Brahmer J. R., Gettinger S. N., Smith D. C., McDermott D. F., Powderly J. D., Carvajal R. D., Sosman J. A., Atkins M. B., Leming P. D., Spigel D. R., Antonia S. J., Horn L., Drake C. G., Pardoll D. M., Chen L., Sharfman W. H., Anders R. A., Taube J. M., McMiller T. L., Xu H., Korman A. J., Jure-Kunkel M., Agrawal S., McDonald D., Kollia G. D., Gupta A., Wigginton J. M., and Sznol M. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J. R., Tykodi S. S., Chow L. Q., Hwu W. J., Topalian S. L., Hwu P., Drake C. G., Camacho L. H., Kauh J., Odunsi K., Pitot H. C., Hamid O., Bhatia S., Martins R., Eaton K., Chen S., Salay T. M., Alaparthy S., Grosso J. F., Korman A. J., Parker S. M., Agrawal S., Goldberg S. M., Pardoll D. M., Gupta A., and Wigginton J. M. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romagné F., André P., Spee P., Zahn S., Anfossi N., Gauthier L., Capanni M., Ruggeri L., Benson D. M. Jr., Blaser B. W., Della Chiesa M., Moretta A., Vivier E., Caligiuri M. A., Velardi A., and Wagtmann N. (2009) Preclinical characterization of 1–7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 114, 2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godal R., Bachanova V., Gleason M., McCullar V., Yun G. H., Cooley S., Verneris M. R., McGlave P. B., and Miller J. S. (2010) Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol. Blood Marrow Transplant. 16, 612–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarek N., Le Luduec J. B., Gallagher M. M., Zheng J., Venstrom J. M., Chamberlain E., Modak S., Heller G., Dupont B., Cheung N. K., and Hsu K. C. (2012) Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J. Clin. Investig. 122, 3260–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao X. W., van Beek E. M., Schornagel K., Van der Maaden H., Van Houdt M., Otten M. A., Finetti P., Van Egmond M., Matozaki T., Kraal G., Birnbaum D., van Elsas A., Kuijpers T. W., Bertucci F., and van den Berg T. K. (2011) CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc. Natl. Acad. Sci. U.S.A. 108, 18342–18347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majeti R., Chao M. P., Alizadeh A. A., Pang W. W., Jaiswal S., Gibbs K. D. Jr., van Rooijen N., and Weissman I. L. (2009) CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138, 286–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao M. P., Alizadeh A. A., Tang C., Jan M., Weissman-Tsukamoto R., Zhao F., Park C. Y., Weissman I. L., and Majeti R. (2011) Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 71, 1374–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao M. P., Jaiswal S., Weissman-Tsukamoto R., Alizadeh A. A., Gentles A. J., Volkmer J., Weiskopf K., Willingham S. B., Raveh T., Park C. Y., Majeti R., and Weissman I. L. (2010) Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2, 63ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao M. P., Alizadeh A. A., Tang C., Myklebust J. H., Varghese B., Gill S., Jan M., Cha A. C., Chan C. K., Tan B. T., Park C. Y., Zhao F., Kohrt H. E., Malumbres R., Briones J., Gascoyne R. D., Lossos I. S., Levy R., Weissman I. L., and Majeti R. (2010) Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142, 699–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willingham S. B., Volkmer J. P., Gentles A. J., Sahoo D., Dalerba P., Mitra S. S., Wang J., Contreras-Trujillo H., Martin R., Cohen J. D., Lovelace P., Scheeren F. A., Chao M. P., Weiskopf K., Tang C., Volkmer A. K., Naik T. J., Storm T. A., Mosley A. R., Edris B., Schmid S. M., Sun C. K., Chua M. S., Murillo O., Rajendran P., Cha A. C., Chin R. K., Kim D., Adorno M., Raveh T., Tseng D., Jaiswal S., Enger P. Ø., Steinberg G. K., Li G., So S. K., Majeti R., Harsh G. R., van de Rijn M., Teng N. N., Sunwoo J. B., Alizadeh A. A., Clarke M. F., and Weissman I. L. (2012) The CD47-signal regulatory protein α (SIRPα) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. U.S.A. 109, 6662–6667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox R. A., Ansell S. M., Lim M. S., Zou W., and Chen L. (2012) The B7 Homologues and their receptors in hematologic malignancies. Eur. J. Haematol. 88, 465–475 [DOI] [PubMed] [Google Scholar]
- 15.Chapoval A. I., Ni J., Lau J. S., Wilcox R. A., Flies D. B., Liu D., Dong H., Sica G. L., Zhu G., Tamada K., and Chen L. (2001) B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat. Immunol. 2, 269–274 [DOI] [PubMed] [Google Scholar]
- 16.Steinberger P., Majdic O., Derdak S. V., Pfistershammer K., Kirchberger S., Klauser C., Zlabinger G., Pickl W. F., Stöckl J., and Knapp W. (2004) Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J. Immunol. 172, 2352–2359 [DOI] [PubMed] [Google Scholar]
- 17.Sun M., Richards S., Prasad D. V., Mai X. M., Rudensky A., and Dong C. (2002) Characterization of mouse and human B7-H3 genes. J. Immunol. 168, 6294–6297 [DOI] [PubMed] [Google Scholar]
- 18.Suh W. K., Gajewska B. U., Okada H., Gronski M. A., Bertram E. M., Dawicki W., Duncan G. S., Bukczynski J., Plyte S., Elia A., Wakeham A., Itie A., Chung S., Da Costa J., Arya S., Horan T., Campbell P., Gaida K., Ohashi P. S., Watts T. H., Yoshinaga S. K., Bray M. R., Jordana M., and Mak T. W. (2003) The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 4, 899–906 [DOI] [PubMed] [Google Scholar]
- 19.Castriconi R., Dondero A., Augugliaro R., Cantoni C., Carnemolla B., Sementa A. R., Negri F., Conte R., Corrias M. V., Moretta L., Moretta A., and Bottino C. (2004) Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc. Natl. Acad. Sci. U.S.A. 101, 12640–12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth T. J., Sheinin Y., Lohse C. M., Kuntz S. M., Frigola X., Inman B. A., Krambeck A. E., McKenney M. E., Karnes R. J., Blute M. L., Cheville J. C., Sebo T. J., and Kwon E. D. (2007) B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 67, 7893–7900 [DOI] [PubMed] [Google Scholar]
- 21.Zang X., Thompson R. H., Al-Ahmadie H. A., Serio A. M., Reuter V. E., Eastham J. A., Scardino P. T., Sharma P., and Allison J. P. (2007) B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. U.S.A. 104, 19458–19463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crispen P. L., Sheinin Y., Roth T. J., Lohse C. M., Kuntz S. M., Frigola X., Thompson R. H., Boorjian S. A., Dong H., Leibovich B. C., Blute M. L., and Kwon E. D. (2008) Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin. Cancer Res. 14, 5150–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boorjian S. A., Sheinin Y., Crispen P. L., Farmer S. A., Lohse C. M., Kuntz S. M., Leibovich B. C., Kwon E. D., and Frank I. (2008) T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin. Cancer Res. 14, 4800–4808 [DOI] [PubMed] [Google Scholar]
- 24.Zang X., Sullivan P. S., Soslow R. A., Waitz R., Reuter V. E., Wilton A., Thaler H. T., Arul M., Slovin S. F., Wei J., Spriggs D. R., Dupont J., and Allison J. P. (2010) Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod. Pathol. 23, 1104–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemke D., Pfenning P. N., Sahm F., Klein A. C., Kempf T., Warnken U., Schnölzer M., Tudoran R., Weller M., Platten M., and Wick W. (2012) Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin Cancer Res. 18, 105–117 [DOI] [PubMed] [Google Scholar]
- 26.Wang L., Zhang Q., Chen W., Shan B., Ding Y., Zhang G., Cao N., Liu L., and Zhang Y. (2013) B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One 8, e70689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamato I., Sho M., Nomi T., Akahori T., Shimada K., Hotta K., Kanehiro H., Konishi N., Yagita H., and Nakajima Y. (2009) Clinical importance of B7-H3 expression in human pancreatic cancer. Br. J. Cancer 101, 1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregorio A., Corrias M. V., Castriconi R., Dondero A., Mosconi M., Gambini C., Moretta A., Moretta L., and Bottino C. (2008) Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology 53, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X., Vale M., Leung E., Kanwar J. R., Gupta R., and Krissansen G. W. (2003) Mouse B7-H3 induces antitumor immunity. Gene Ther. 10, 1728–1734 [DOI] [PubMed] [Google Scholar]
- 30.Wu C. P., Jiang J. T., Tan M., Zhu Y. B., Ji M., Xu K. F., Zhao J. M., Zhang G. B., and Zhang X. G. (2006) Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J. Gastroenterol. 12, 457–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loos M., Hedderich D. M., Ottenhausen M., Giese N. A., Laschinger M., Esposito I., Kleeff J., and Friess H. (2009) Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer 9, 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmeyer K. A., Ray A., and Zang X. (2008) The contrasting role of B7-H3. Proc. Natl. Acad. Sci. U.S.A. 105, 10277–10278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigdorovich V., Ramagopal U. A., Lázár-Molnár E., Sylvestre E., Lee J. S., Hofmeyer K. A., Zang X., Nathenson S. G., and Almo S. C. (2013) Structure and T cell inhibition properties of B7 family member, B7-H3. Structure 21, 707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z., Luther N., Ibrahim G. M., Hawkins C., Vibhakar R., Handler M. H., and Souweidane M. M. (2013) B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J. Neurooncol. 111, 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calabrò L., Sigalotti L., Fonsatti E., Bertocci E., Di Giacomo A. M., Danielli R., Cutaia O., Colizzi F., Covre A., Mutti L., Natali P. G., and Maio M. (2011) Expression and regulation of B7-H3 immunoregulatory receptor, in human mesothelial and mesothelioma cells: immunotherapeutic implications. J. Cell. Physiol. 226, 2595–2600 [DOI] [PubMed] [Google Scholar]
- 36.Modak S., Kramer K., Gultekin S. H., Guo H. F., and Cheung N. K. (2001) Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 61, 4048–4054 [PubMed] [Google Scholar]
- 37.Xu H., Cheung I. Y., Guo H. F., and Cheung N. K. (2009) MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 69, 6275–6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Modak S., Guo H. F., Humm J. L., Smith-Jones P. M., Larson S. M., and Cheung N. K. (2005) Radioimmunotargeting of human rhabdomyosarcoma using monoclonal antibody 8H9. Cancer Biother. Radiopharm. 20, 534–546 [DOI] [PubMed] [Google Scholar]
- 39.Luther N., Cheung N. K., Dunkel I. J., Fraser J. F., Edgar M. A., Gutin P. H., and Souweidane M. M. (2008) Intraparenchymal and intratumoral interstitial infusion of anti-glioma monoclonal antibody 8H9. Neurosurgery 63, 1166–1174 [DOI] [PubMed] [Google Scholar]
- 40.Juhl H., Petrella E. C., Cheung N. K., Bredehorst R., and Vogel C. W. (1997) Additive cytotoxicity of different monoclonal antibody-cobra venom factor conjugates for human neuroblastoma cells. Immunobiology 197, 444–459 [DOI] [PubMed] [Google Scholar]
- 41.Onda M., Wang Q. C., Guo H. F., Cheung N. K., and Pastan I. (2004) In vitro and in vivo cytotoxic activities of recombinant immunotoxin 8H9(Fv)-PE38 against breast cancer, osteosarcoma, and neuroblastoma. Cancer Res. 64, 1419–1424 [DOI] [PubMed] [Google Scholar]
- 42.Luther N., Cheung N. K., Souliopoulos E. P., Karampelas I., Bassiri D., Edgar M. A., Guo H. F., Pastan I., Gutin P. H., and Souweidane M. M. (2010) Interstitial infusion of glioma-targeted recombinant immunotoxin 8H9scFv-PE38. Mol. Cancer Ther. 9, 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung N. K., Guo H. F., Modak S., and Cheung I. Y. (2003) Anti-idiotypic antibody facilitates scFv chimeric immune receptor gene transduction and clonal expansion of human lymphocytes for tumor therapy. Hybrid Hybridomics 22, 209–218 [DOI] [PubMed] [Google Scholar]
- 44.Kramer K., Humm J. L., Souweidane M. M., Zanzonico P. B., Dunkel I. J., Gerald W. L., Khakoo Y., Yeh S. D., Yeung H. W., Finn R. D., Wolden S. L., Larson S. M., and Cheung N. K. (2007) Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J. Clin. Oncol. 25, 5465–5470 [DOI] [PubMed] [Google Scholar]
- 45.He P., Kramer K., Smith-Jones P., Zanzonico P., Humm J., Larson S. M., and Cheung N. K. (2011) Two-compartment model of radioimmunotherapy delivered through cerebrospinal fluid. Eur. J. Nucl. Med. Mol. Imaging 38, 334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kramer K., Kushner B. H., Modak S., Pandit-Taskar N., Smith-Jones P., Zanzonico P., Humm J. L., Xu H., Wolden S. L., Souweidane M. M., Larson S. M., and Cheung N. K. (2010) Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J. Neurooncol. 97, 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loo D., Alderson R. F., Chen F. Z., Huang L., Zhang W., Gorlatov S., Burke S., Ciccarone V., Li H., Yang Y., Son T., Chen Y., Easton A. N., Li J. C., Rillema J. R., Licea M., Fieger C., Liang T. W., Mather J. P., Koenig S., Stewart S. J., Johnson S., Bonvini E., and Moore P. A. (2012) Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin. Cancer Res. 18, 3834–3845 [DOI] [PubMed] [Google Scholar]
- 48.Cheung I. Y., Farazi T. A., Ostrovnaya I., Xu H., Tran H., Mihailovic A., Tuschl T., and Cheung N. K. (2014) Deep MicroRNA sequencing reveals downregulation of miR-29a in neuroblastoma central nervous system metastasis. Genes Chromosomes Cancer 53, 803–814 [DOI] [PubMed] [Google Scholar]
- 49.Vagin A., and Teplyakov A. (2010) Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66, 22–25 [DOI] [PubMed] [Google Scholar]
- 50.Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., and Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones T. A., Zou J. Y., Cowan S. W., and Kjeldgaard M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 [DOI] [PubMed] [Google Scholar]
- 52.Chen R., Li L., and Weng Z. (2003) ZDOCK: an initial-stage protein-docking algorithm. Proteins 52, 80–87 [DOI] [PubMed] [Google Scholar]
- 53.Sánchez R., and Sali A. (1997) Evaluation of comparative protein structure modeling by MODELLER-3. Proteins Suppl. 1, 50–58 [DOI] [PubMed] [Google Scholar]
- 54.Rocchia W., Sridharan S., Nicholls A., Alexov E., Chiabrera A., and Honig B. (2002) Rapid grid-based construction of the molecular surface and the use of induced surface charge to calculate reaction field energies: applications to the molecular systems and geometric objects. J. Comput. Chem. 23, 128–137 [DOI] [PubMed] [Google Scholar]
- 55.Cheung N. K., Guo H., Hu J., Tassev D. V., and Cheung I. Y. (2012) Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology 1, 477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Q., Feng Y., Zhu Z., and Dimitrov D. S. (2011) Human monoclonal antibody fragments binding to insulin-like growth factors I and II with picomolar affinity. Mol. Cancer Ther. 10, 1677–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Q., Ahmed M., Tassev D. V., Hasan A., Kuo T. Y., Guo H. F., O'Reilly R. J., and Cheung N. K. (2015) Affinity maturation of T-cell receptor-like antibodies for Wilms tumor 1 peptide greatly enhances therapeutic potential. Leukemia 29, 2238–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., and Lowry P. J. (1981) Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3α,6α-diphenyl glycoluril (Iodogen). Anal. Biochem. 117, 136–146 [DOI] [PubMed] [Google Scholar]
- 59.Harlow E., and Lane D. (1999) Using Antibodies: a Laboratory Manual, pp. 330–331, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 60.Ahmed M., Hu J., and Cheung N. K. (2014) Structure based refinement of a humanized monoclonal antibody that targets tumor antigen disialoganglioside GD2. Front. Immunol. 5, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Q., Ahmed M., Guo H. F., Cheung I. Y., and Cheung N. K. (2015) Alteration of electrostatic surface potential enhances affinity and tumor killing properties of anti-ganglioside GD2 monoclonal antibody hu3F8. J. Biol. Chem. 290, 13017–13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin D. Y., Tanaka Y., Iwasaki M., Gittis A. G., Su H. P., Mikami B., Okazaki T., Honjo T., Minato N., and Garboczi D. N. (2008) The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc. Natl. Acad. Sci. U.S.A. 105, 3011–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lázár-Molnár E., Yan Q., Cao E., Ramagopal U., Nathenson S. G., and Almo S. C. (2008) Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc. Natl. Acad. Sci. U.S.A. 105, 10483–10488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stamper C. C., Zhang Y., Tobin J. F., Erbe D. V., Ikemizu S., Davis S. J., Stahl M. L., Seehra J., Somers W. S., and Mosyak L. (2001) Crystal structure of the B7–1/CTLA-4 complex that inhibits human immune responses. Nature 410, 608–611 [DOI] [PubMed] [Google Scholar]
- 65.Schwartz J. C., Zhang X., Fedorov A. A., Nathenson S. G., and Almo S. C. (2001) Structural basis for co-stimulation by the human CTLA-4/B7–2 complex. Nature 410, 604–608 [DOI] [PubMed] [Google Scholar]