FIGURE 3.
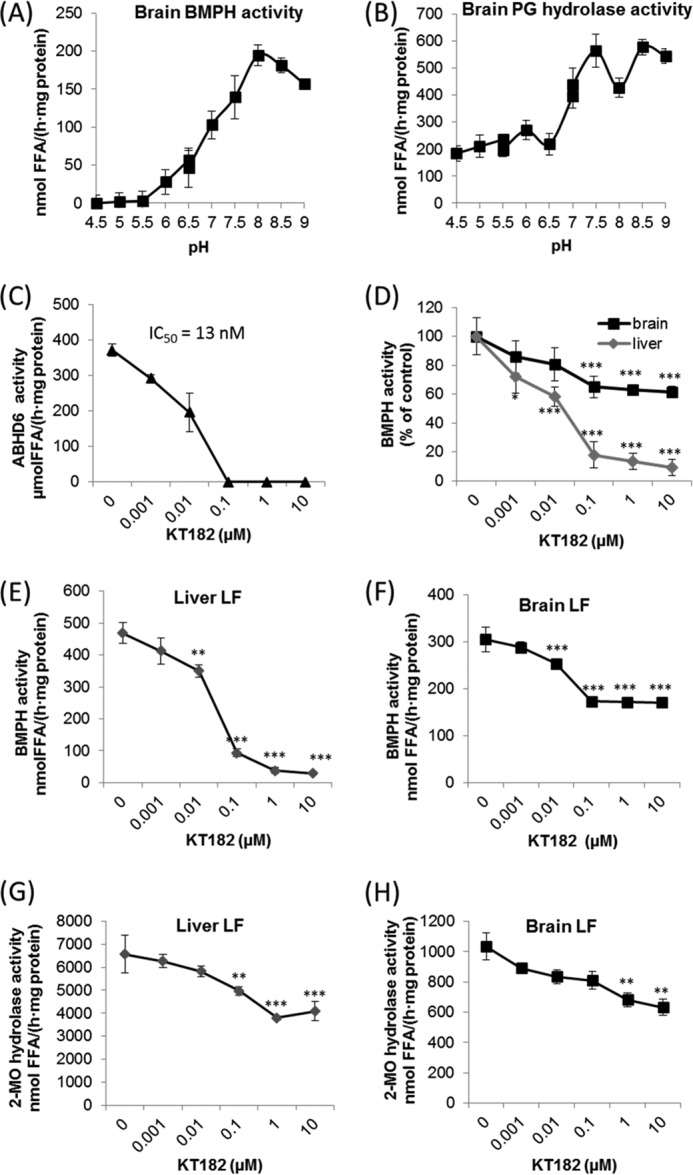
BMP is resistant to lysosomal hydrolases and degraded efficiently in the slightly alkaline pH range in a process involving ABHD6. A and B, pH dependence of (A) BMP and (B) PG hydrolase activity of brain lysates. Degradation of BMP(S,S) and PG (2 mm each) was monitored between pH 4.5 and 9.0 as described in Fig. 1D. C, inhibition of BMPH activity of purified ABHD6 by KT182 using BMP(S,S) as substrate. D, inhibition of brain and liver BMPH activity by KT182. E and F, inhibition of BMPH activity by KT182 in LF of liver and brain. LF were purified as described in Fig. 2B. G and H, inhibition of MG hydrolase activity in LF of liver and brain by KT182. C—H, activity assays were performed at pH 8.0 using BMP(S,S) and 2-monoolein (2-MO) as substrate (2 mm each). Data are presented as mean ± S.D. and represent at least three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; analysis of variance.
