FIGURE 1.
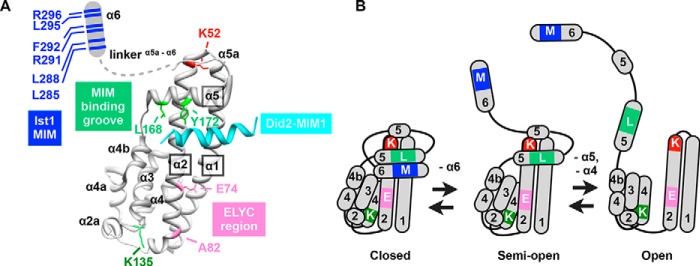
Putative Vps4 regulatory elements in Ist1. A, site-directed mutagenesis of Ist1. Residues mutated in these studies are indicated in the Ist1 NTD-Did2 MIM1 co-crystal structure (PDB code 3GGZ (67)), including the Did2-MIM1 element (cyan), the Ist1 MIM element (blue, Leu285, Leu288, Arg291, Phe292, Leu295, and Arg296), the Did2-MIM1-binding motif (light green, Leu168 and Tyr172), a highly conserved lysine of unknown function (dark green, Lys135), the ELYC region (pink, Glu74 and Ala78), and a highly conserved lysine corresponding to hIST1 (Arg51) that was defective for Ist1 homopolymerization in vitro (45) (red, Lys52). The Ist1 α6 and adjacent linker region are drawn as a schematic in a theoretical semi-open Ist1 conformation. B, model for Ist1 conformational changes. In the closed conformation, α6 is buried within the α1/2/5 groove. In the semi-open conformation, α6 is displaced from the α1/2/5 binding groove. In the open conformation, the α1,2,5 groove is dissolved as α5 is displaced from the closed end of the α1/2 hairpin. Further conformational changes in the Ist1 core domain may occur, including unpacking of the α3/4 bundle from the open end of the α1/2 hairpin. Mutations are indicated in distinct Ist1 conformations: MIM residues, M (α6); MIM-binding motif, L (α5); K135, K (α4); ELYC region, E (α2); and K52, K (α2).