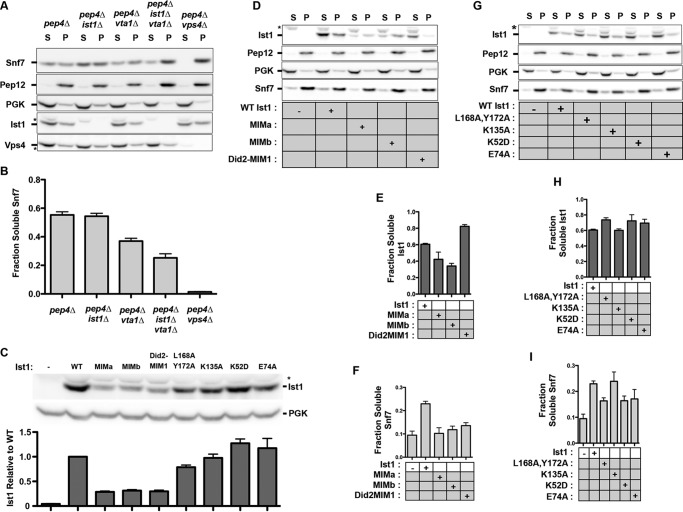
FIGURE 10.
Ist1 conformation leads to altered membrane association in vivo. A, subcellular fractionation of the ESCRT-III subunits Ist1and Snf7 in pepΔ, pepΔ ist1Δ, pepΔ vta1Δ, pep4Δ ist1Δ vta1Δ and pep4Δ vps4Δ yeast strains. Blots are representative of experiments performed four times. Phosphoglycerate kinase and Pep12 served as markers for the 13,000 × g soluble (S) and pellet (P) fractions. Subcellular fractionation of the ATPase Vps4 is also shown. Nonspecific species detected by Ist1 and Vps4 antibodies are indicated (asterisks). B, quantitation of soluble Snf7 in the four experiments is indicated. Error bars indicate mean ± S.D. C, whole cell lysates showing total Ist1 protein levels in pep4Δ ist1Δ vta1Δ, with phosphoglycerate kinase (PGK) shown as a loading control. D and G, subcellular fractionation of ESCRT-III subunits Ist1 and Snf7 in ist1Δ vta1Δ pepΔ yeast with plasmids expressing WT Ist1 and Ist1 mutants. Blots are representative of experiments performed three times. Phosphoglycerate kinase and Pep12 served as markers for the 13,000 × g soluble and pellet fractions. A nonspecific species detected by Ist1 antibody is indicated (asterisks). E—I, quantitation of three experiments. E and H, relative amount of Ist1 in soluble fraction. H, relative amount of Snf7 in soluble fraction. Error bars indicate mean ± S.D.