FIGURE 11.
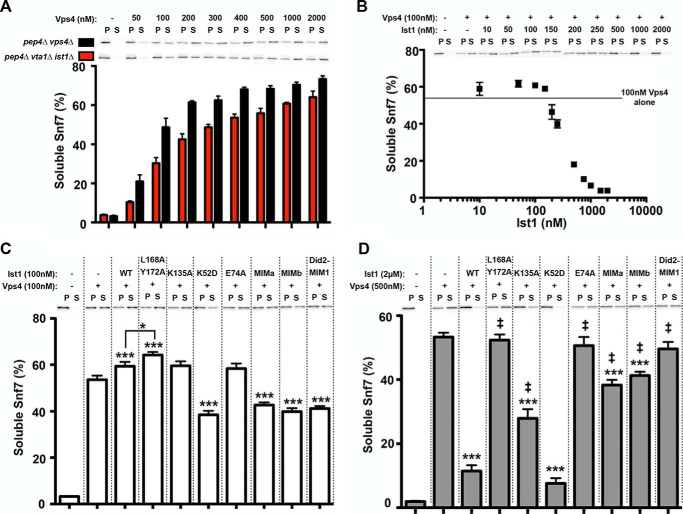
Ist1 exerts positive and negative regulation of ESCRT-III disassembly in vitro. A, Vps4-mediated Snf7 disassembly from pep4Δ vps4Δ (black columns) and pep4Δ vta1Δ ist1Δ (red columns). Vps4 was titrated from 50 nm-2 μm. Representative blots of Snf7 localization to the soluble (S) and pellet (P) fractions are shown. Quantitation of two experiments with reactions performed in duplicate is indicated as percent Snf7 in soluble fraction. Error bars indicate mean ± S.E. B, effects of WT Ist1 on Vps4-mediated Snf7 disassembly from pep4Δ vta1Δ ist1Δ. WT Ist1 was titrated from 10 nm-2 μm in the presence of 100 nm Vps4. Basal Snf7 disassembly by 100 nm Vps4 is indicated by the line to highlight enhanced Snf7 disassembly in the presence of WT Ist1 at low [Ist1], followed by inhibition at high [Ist1]. Representative blots of Snf7 localization to the soluble and pellet fractions are shown. Quantitation of three experiments with reactions performed in duplicate is indicated as percent Snf7 in soluble fraction. Error bars indicate mean ± S.E. C, effects of 100 nm Ist1 mutants on Vps4-mediated Snf7 disassembly at 100 nm Vps4. Representative blots of Snf7 localization to the soluble and pellet fractions are shown. Quantitation of three experiments with reactions performed in triplicate is indicated as percent Snf7 in soluble fraction. Statistical differences from addition of Vps4 alone (***, p < 0.001) or from addition of Vps4 and WT Ist1 are indicated (*, p < 0.05). D, effects of excess (2 μm) Ist1 mutants on Vps4-mediated Snf7 disassembly in the presence of 500 nm Vps4. Representative blots of Snf7 localization to the soluble and pellet fractions are shown. Quantitation of three experiments with reactions performed in triplicate is indicated as percent Snf7 in soluble fraction. Error bars indicate mean ± S.E. Statistical differences from addition of Vps4 alone (***, p < 0.001) or from addition of Vps4 and WT Ist1 are indicated (‡, p < 0.001).