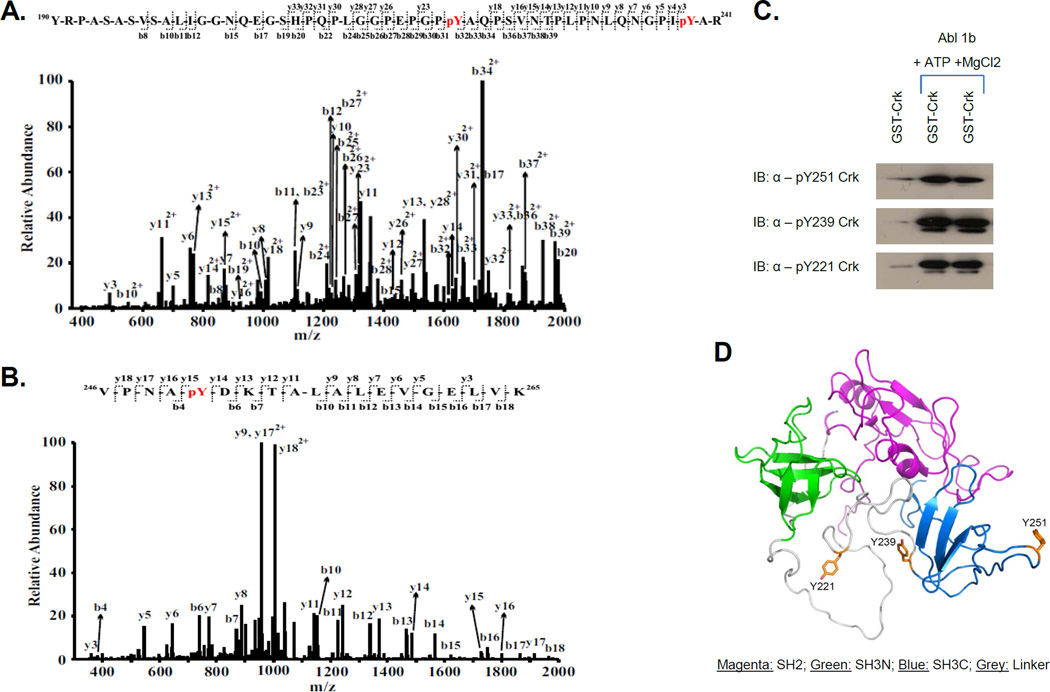
Figure 1. Identification of phosphorylation sites pY221, pY239 and pY251 on Crk by LC-MS/MS.
A, MS/MS spectrum of a quadruply-charged ion (m/z 1371.16) corresponding to a doubly phosphorylated peptide 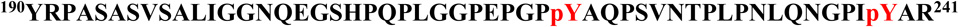 is shown. The phosphorylation sites are Y221 and Y239. B, MS/MS spectrum of a doubly-charged ion (m/z 1055.53) corresponding to the peptide sequence
is shown. The phosphorylation sites are Y221 and Y239. B, MS/MS spectrum of a doubly-charged ion (m/z 1055.53) corresponding to the peptide sequence 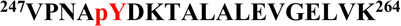 with a phosphorylation modification at Y251 is shown. The observed y- and b-ion series confirmed the peptide sequence and modification. C, GST-Crk was in vitro phosphorylated by immunoprecipitated Abl 1b in a kinase reaction and samples were analyzed by western blotting with anti-pY221 Crk (bottom), anti-pY239 Crk (middle) and anti-pY251 Crk (top) antibodies. D, Locations of Y239 and Y251 on the SH3C and Y221 on the inter-SH3 linker are depicted on the solution structure of Crk (PDB ID: 2EYZ).
with a phosphorylation modification at Y251 is shown. The observed y- and b-ion series confirmed the peptide sequence and modification. C, GST-Crk was in vitro phosphorylated by immunoprecipitated Abl 1b in a kinase reaction and samples were analyzed by western blotting with anti-pY221 Crk (bottom), anti-pY239 Crk (middle) and anti-pY251 Crk (top) antibodies. D, Locations of Y239 and Y251 on the SH3C and Y221 on the inter-SH3 linker are depicted on the solution structure of Crk (PDB ID: 2EYZ).