Fig. 1.
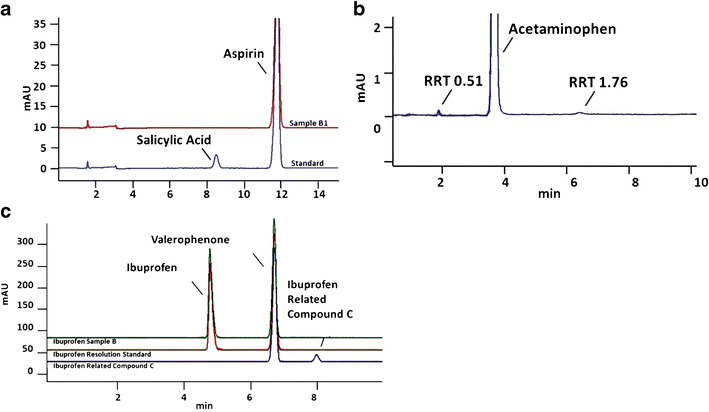
Representative chromatograms from analysis of pain-relief medications. a A commercial standard that includes a common contaminant, salicylic acid, is shown in the lower trace for comparison, with the free salicylic acid peak labeled at ∼RT = 8.5 min. Results from analysis of sample B1 are shown superimposed. Note the absence of a salicylic acid peak in the aged medication sample. Aspirin samples 9 months beyond their original manufacturer expiration date were found to contain active ingredient well within USP guidelines (96.5% of the label claim of 325 mg per tablet). Free salicylic acid was found at less than 0.05% in each sample; the USP limit for this impurity is no more than 0.3%. b Acetaminophen samples that were analyzed 5 months after their original manufacturer expiration date were found to contain active ingredient at 96.5% of the label claim of 325 mg per tablet, which is within the USP at the time these samples were analyzed, but outside the new USP requirements of 98–102%. Although two additional peaks were observed, 0.17 and 0.25% (by peak area), they were not identified. If one of them was paraaminophenol, this sample would also have exceeded the limit on this impurity (NMT 0.005%). c Ibuprofen is known to have several potential ingredients in addition to the active ingredient, so multiple standards were run for comparison. Ibuprofen samples analyzed 3 months prior to their original expiration date also met USP criteria with active ingredient well within USP guidelines, 99.9% of the label claim of 400 mg per tablet. USP has identified and determined limits for an impurity in ibuprofen, called ibuprofen related compound C. Ibuprofen related compound C was found at less than 0.001% in each sample; the USP limit for this impurity is 0.25%
