Fig. 2.
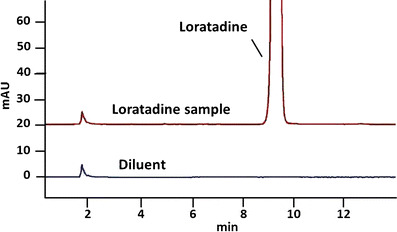
Representative chromatograms from analysis of antihistamine loratadine. Antihistaminergic loratadine samples were found to contain active ingredient well within USP guidelines at 8 months beyond their manufacturer’s expiration date, 99.9% of the label claim of 10 mg per tablet. The USP-identified impurity 4-(8-chloro-11-fluoro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine-11-yl)-1-piperidinecarboxylate ethyl (RRT 0.79) was not detected
