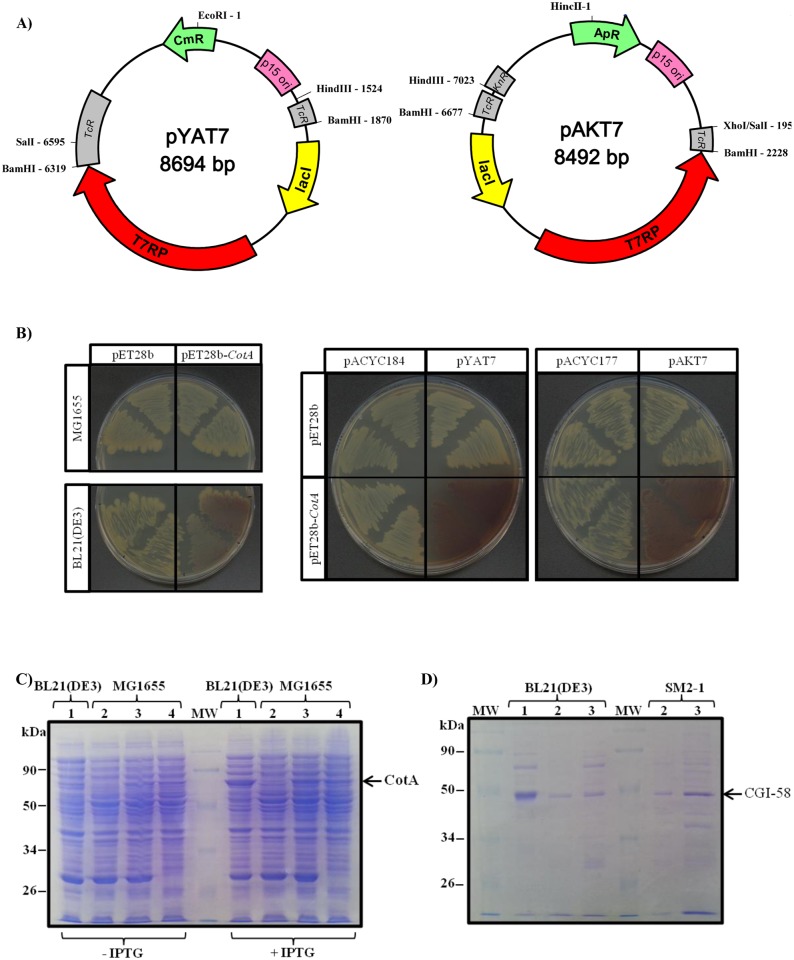
Fig 1. A new set of plasmids containing T7 RNA polymerase and with a replication origin compatible with the pET plasmid series, allowing expression and purification of recombinant proteins in E. coli.
A) Maps of vectors pYAT7 and pAKT7 containing T7 RNA polymerase cloned into plasmids harboring the p15A origin of replication. Left panel: pYAT7 plasmid with T7RP cloned into the BamHI site of the pACYC184 backbone, thus conferring resistance to chloramphenicol. Right panel: pAKT7 plasmid with T7RP cloned into the HindIII-SalI sites of the pACYC177 backbone, thus conferring resistance to ampicillin. Active and inactive antibiotic resistance genes are shown in green and in grey, respectively. Remarkable restriction sites are shown. B) Functional use of pYAT7 and pAKT7 plasmids for expressing the recombinant CotA protein in MG1655, a T7RP deficient E. coli strain. Left panel: coloration due to ABTS oxidation in different E. coli genetic backgrounds necessitates both the presence of CotA and T7RP. MG1655 or BL21(DE3) strains were either transformed with void pET28b or with pET28b-CotA. Central and right panels: functional expression of CotA in MG1655 strain transformed with either pYAT7, pAKT7 plasmids or native pACYC184 or pACYC177 plasmids. LB plates contain 250 μM CuSO4, 1 mM ABTS and 1 μM IPTG. All pictures were taken 3 days after streaking. C) Functional expression of CotA cloned into pYAT7 and pAKT7 plasmids with analysis by SDS-PAGE of total proteins extracted from the recombinant strain. Extracts were analyzed before or after a 3 h induction with IPTG. Lane 1: strain BL21(DE3) transformed with pET28b-CotA. Lane 2 and 3: strain MG1655 transformed with pYAT7 and either void plasmid (lane 2) or pET28b-CotA (lane 3). Lane 4: strain MG1655 transformed with pAKT7 and pET28b-CotA. The expected size of CotA (58.5 kDa) is indicated. D) Analysis by SDS-PAGE of purified CGI-58, from plant or mouse, expressed either in BL21(DE3) strain or SM2-1 and transformed with pYAT7. 5 μg of recombinant MmCGI-58 and 2 μg of recombinant plant CGI-58 were loaded onto each lane. Lane 1: MmCGI-58 (42.0 kDa), Lane 2: truncated version of AtCGI-58 (44.5 kDa), Lane 3: full-length version of AtCGI-58 (47.8 kDa). The authenticity of purified recombinant MmCGI-58 was verified by mass spectrometry after tryptic digestion, and for recombinant plant CGI-58 a Western-blot with monoclonal Anti-polyHistidine antibody was performed.