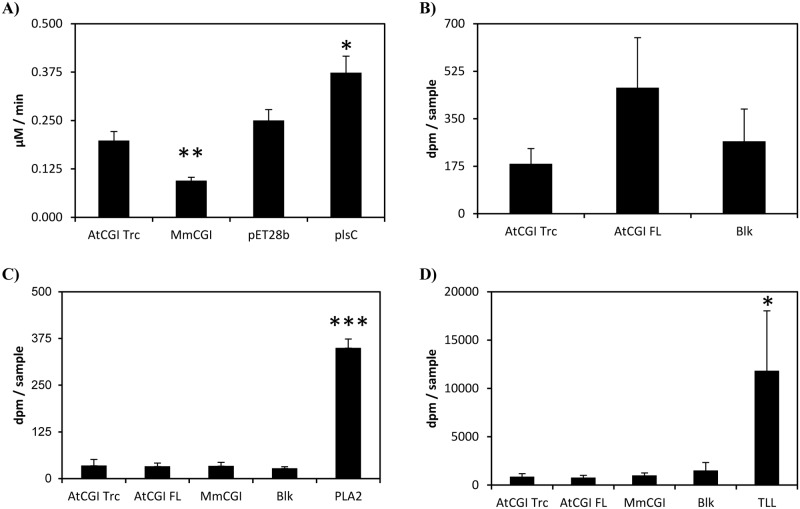
Fig 3. Reassessing the potential activities of recombinant plant CGI-58 using enzymatic tests.
A) Measurement of PA formation, through LPAAT activity, using 5 μg of protein from a crude extract of SM2-1 cells transformed either with the void pET28b vector, or the truncated version of AtCGI-58, or MmCGI-58 or plsC. Activities were corrected from the blank values obtained with samples tested in the absence of protein; blank values represent less than 10% of the dpm counted with other samples. Values ± SD are the mean of three independent measurements. *P<0.05, ** P<0.01 (vs pET28b). The experiment was repeated with similar results. B) Measurement of total dpm found in the PA fraction after TLC separation with purified recombinant plant CGI-58. LPAAT activity was tested with samples incubated for 10 min in the presence of 1 μg of a purified full-length or truncated version of AtCGI-58 expressed in the SM2-1 strain. Blanks (Blk) were prepared with no protein incubation. Values ± SD are the mean of three independent measures. The experiment was repeated with similar results. C) Measurement of the phospholipase activity of purified recombinant plant CGI-58. (D) Measurement of the triglyceride lipase activity of purified recombinant plant CGI-58. For both figures, the total dpm counted in the free fatty acid fraction after TLC separation is shown. Samples were incubated for 40 min in the presence of 1 μg of a purified full-length or truncated version of AtCGI-58 expressed in the SM2-1 strain. Porcine pancreatic phospholipase A2 (1 ng) and triglyceride lipase from Thermomyces lanuginosus (TLL) (100 ng) were used as positive controls. Blanks (Blk) were prepared with no protein incubation. Values ± SD are the mean of three independent measures. * P<0.05, *** P<0.001 (vs blank). The experiment was repeated with similar results.