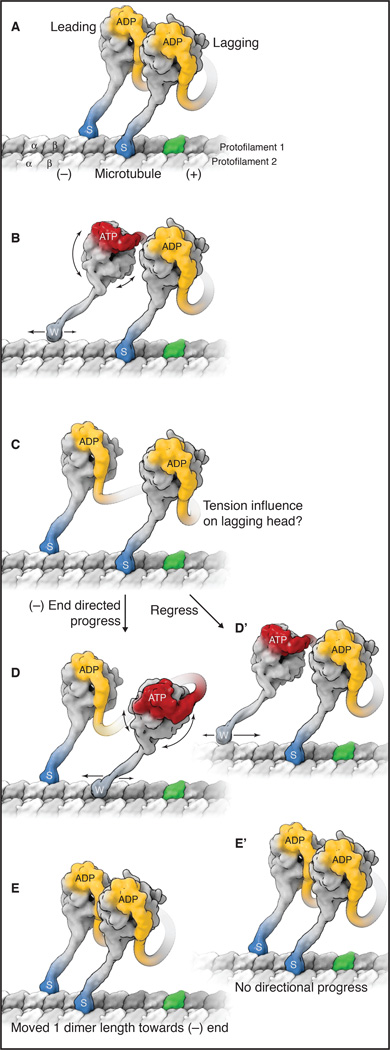
Figure 2.
A model for dynein stepping along a microtubule. See also Movie S1. A) The sequence starts with a cytoplasmic dynein dimer with ADP bound in both motor domains (yellow) and both microtubule-binding domains (MTBDs) tightly bound to the microtubule, in the strong-binding state (indicated by “s” and darker blue color). One tubulin dimer in the microtubule is highlighted in green to allow easier identification of dynein progression towards the minus end. B) When ADP is exchanged for ATP in the leading head (red), conformational changes occur in the ring, which are communicated to the MTBD via a change in registry of the coiled-coil stalk, thus releasing the MTBD from the microtubule. This sequence triggers the reposition of the N terminus of the linker over AAA2/3 and a resultant rotation of the ring/MTBD and a slight forward re-positioning of the MTBD. The weakly bound motor domain (indicated by “w” and lighter blue color) is also subject to Brownian motion, which causes a two-dimensional search of the MTBD on top of the microtubule lattice. The weak-to-strong binding transition is favored with the stalk pointing backwards, and this locks a forward step in place by the front head (see also Figure 3). This asymmetry in the binding properties of the MTBD provides an additional proof-reading mechanism that favors a forward versus a backward step. C) This strong rebinding triggers the movement of the linker from AAA2/3 to AAA4/5. This minus-end directed shift of the N terminus of the linker reduces the slack in the connection between the motor domains and applies tension to the lagging motor domain. At this point, two potential subsequent steps are shown (D,E and D',E'). D and E) The rear head now detaches after ADP/ATP exchange and undergoes the same sequence of events described for the front head, again resulting in a step forward towards the front head and relieving the tension between the two motor domains. D’ and E’) The leading motor domain undergoes ADP/ATP exchange and detaches, but tension between the motor domains overrides the forward biasing mechanisms and the leading MTBD steps backward. In this instance, the homodimer does not undergo a net displacement from A to E’.