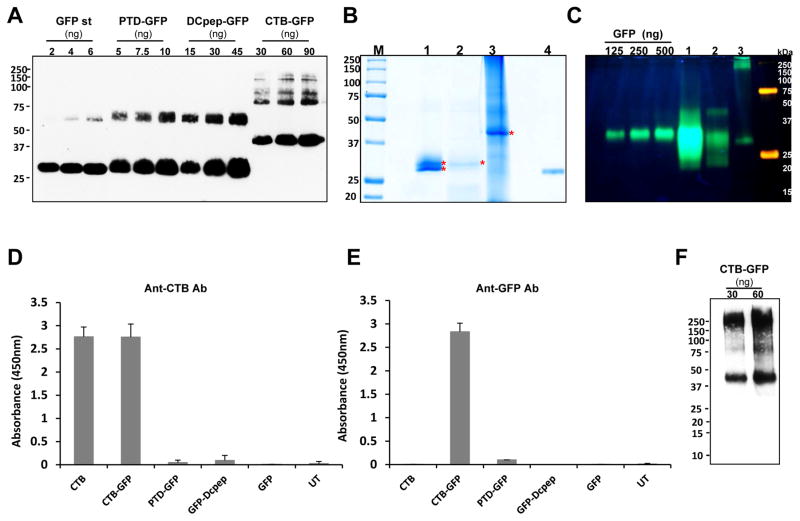
Fig. 4. Characterization of purified GFP fused proteins.
(A) Quantification of purified GFP fused proteins, and coomassie staining and fluorescence image. Densitometric assay with western blot image was done with known amount of GFP standard protein to quantify the purified tag-fused GFP proteins. Purified proteins were run on SDS- PAGE and immunoprobed with anti-GFP antibody. Loading amounts were indicated as shown. Purity was calculated as a percentage of the amount detected on the immunoblot assay to total loading amount. (B) Coomassie staining of purified GFP tagged proteins. M, protein molecular weight marker; lane 1, PTD-GFP (10 μL, 2.37 μg); lane 2, Dcpep-GFP (40 μL, 3.12 μg), lane 3, CTB-GFP (10 μL, 32.8 μg), and lane 4, GFP (400 ng). (C) Non-denaturing SDS-PAGE of purified GFP fusion proteins in order to determine GFP fluorescence. Lane 1 (PTD-GFP 10μl, 9.17μg TSP loading), lane 2 (DCpep-GFP 15μl, 4.6μg TSP loading) and lane 3 (CTB-GFP 20μl, 33μg TSP loading). (D and E) The purified GFP-tagged proteins were examined for their binding affinity to GM1 receptor. Anti-CTB (D) and anti-GFP (E) antibody were used to detect the interaction between GM1 and the GFP fusion proteins. The protein amounts used for the assay are as follows. CTB, 10 pg; CTB-GFP, 1.25 ng; PTD-GFP 10ng; DCpep-GFP 10ng; GFP, 10ng and UT, untransformed wild type total proteins, 100ng. (F) Non-denaturing Tris-tricine PAGE of purified CTB-GFP to determine pentameric structure. Pentameric structure of purified CTB-GFP was immunoprobed using anti-CTB antibody (1 in 10,000). Loading amounts of CTB-GFP are indicated as in the figure.