Fig. 5. in vitro release profile and stability of drug-loaded exosomes.
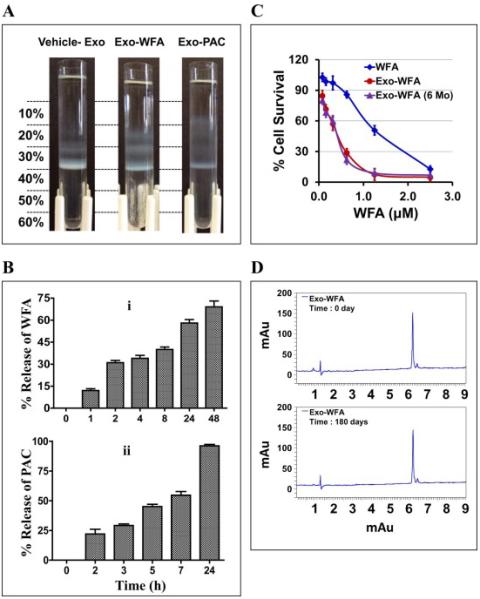
(A) Separation of vehicle- and drug-loaded milk exosomes by Opti-prep density gradient. Indicated agents were mixed with exosome in the presence of 10% ethanol. Unbound drug was removed by centrifugation at 10,000×g for 10 min. The drug loaded exosomes were layered onto Opti-prep gradient (10 - 60%) and separated by centrifugation at 150,000×g for 16 h using SW 41 Ti swing rotor. (B) The release study was done of Exo-WFA (top) and Exo- PAC (bottom) formulations was using dialysis tubes against buffer containing the surfactant, Tween-80 (0.02%) at 37°C. (C) Anti-proliferative activity of exosomal-WFA formulation of fresh preparation (red) and after 6 months of storage (purple) against A549 cells. Free WFA was included for reference (blue) (mean ± SD). (D) UPLC profile of WFA extracted from exosomal formulation at day 0 and after 180 days.
