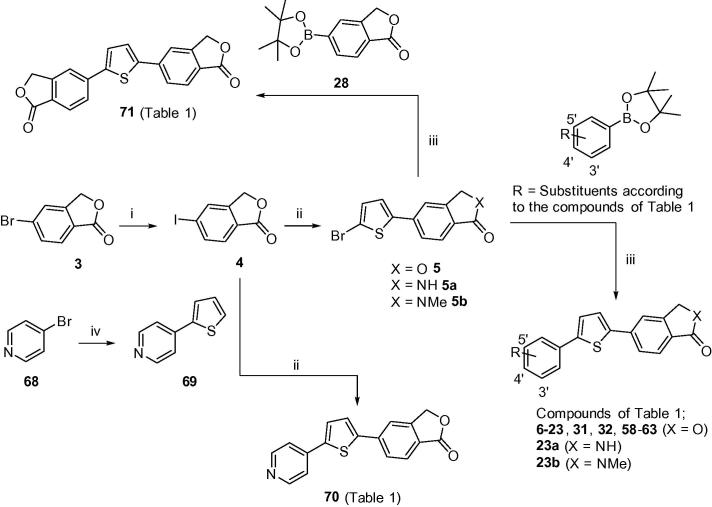
Scheme 1.
Reagents and conditions: (i) sealed tube, 1,4-dioxane, CuI (5.0 mol %), NaI, racemic trans-N,N′-dimethyl-1,2-cyclohexanediamine (10 mol %), 110 °C, N2, 18 h; (ii) 2-bromothiophene or 69, DMSO, KF, PdCl2(PPh3)2, AgNO3, 100 °C, N2, 2 h; (iii) toluene/EtOH, 2 M Na2CO3, PdCl2(dppf)·CH2Cl2, 85–90 °C, N2, 2–6 h; (iv) thiophene-2-boronic acid, toluene/EtOH, 2 M Na2CO3, PdCl2(dppf)·CH2Cl2, 85 °C, N2, 2 h.