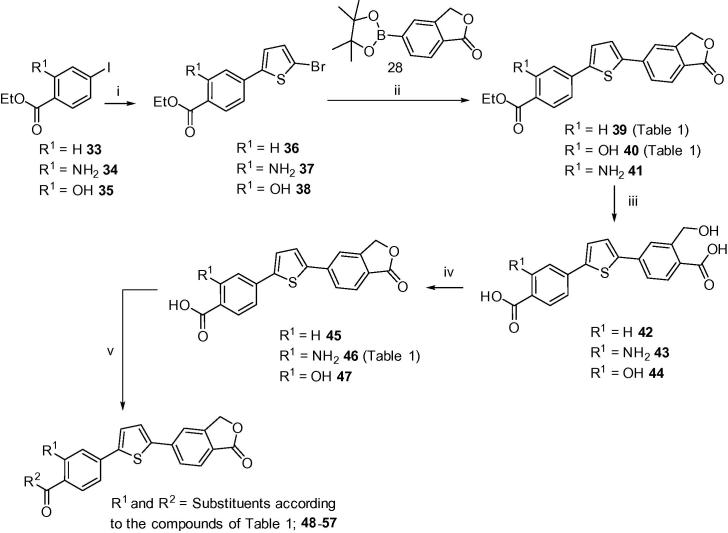
Scheme 3.
Reagents and conditions: (i) 2-bromothiophene, DMSO, KF, PdCl2(PPh3)2, AgNO3, 100 °C, N2, 2–4 h; (ii) toluene/EtOH, 2 M Na2CO3, PdCl2(dppf)·CH2Cl2, 85–90 °C, N2, 1–6 h; (iii) MeOH, 2 M NaOH, 100 °C, 2 h, HCl-workup; (iv) TFA–DCM (1:1), rt, 3–4 h; (v) THF, pyridine, pentafluorophenyltrifluoroacetate, rt, 2 h, R2-NH2, rt, 2 h, 48; or pyridine, DCC, HOBt, 75 °C, 5–6 h, 49–54; or anhydrous-DMF, EDCI·HCl, HOBt, rt, 2 h, R2-NH2, 0 °C → rt, 1–2 h, 55–57.