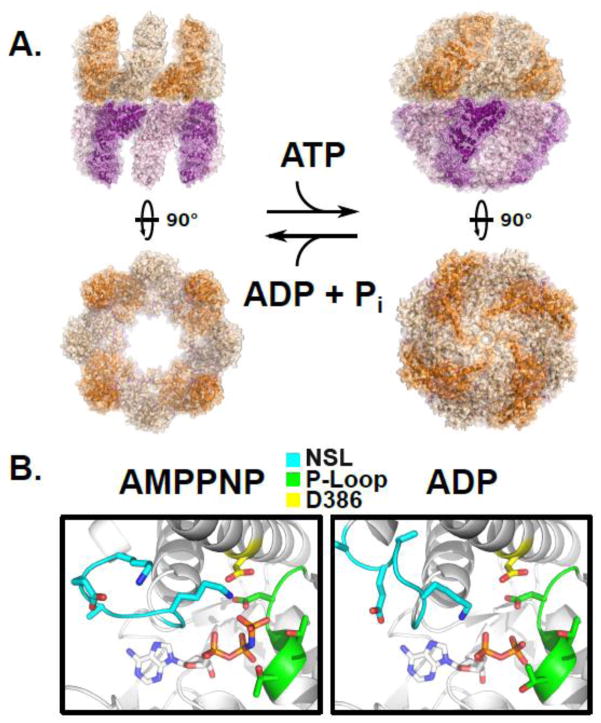
Figure 4.
The ATP driven conformational cycle of the group II chaperonin. A) Left, the open apo state of a group II chaperonin MmCpn determined by cryoelectron microscopy, PDBID: 3IYF. Right, the cryoelectron microscopy structure of wild type MmCpn in the ATP-induced closed state, PDBID: 3LOS [64]. B) The position of the nucleotide sensing loop (NSL) is dependent upon the presence of the γ-phosphate. Left, the crystal structure of the archaeal chaperonin MmCpn in a pre-hydrolysis state, complexed with the non-hydrolyzable ATP analog AMPPNP, PDBID: 3RUV. Right, the crystal structure of the same MmCpn complexed with ADP, PDBID: 3RUW [40]. The nucleotide sensing loop, P-loop, and catalytic aspartate, D386 are indicated in cyan, green, and yellow respectively. The conformation of the NSL is altered by the scission of the gamma phosphate between the left and right panels.