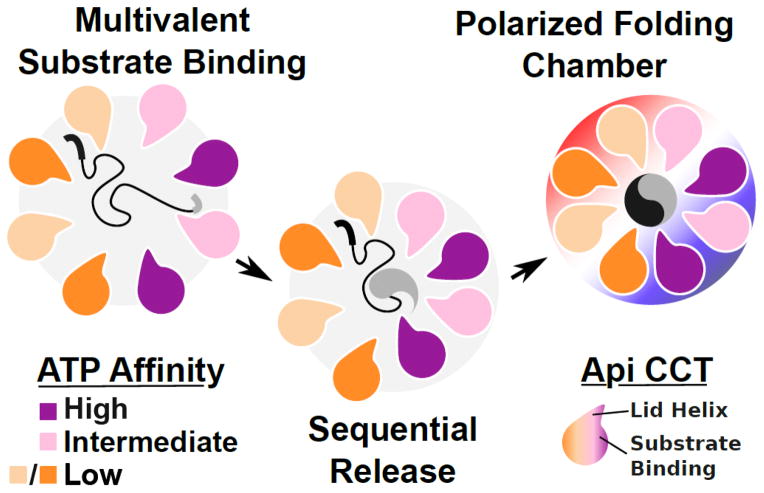
Figure 5.
A schematic view of factors promoting substrate folding by TRiC and other group II chaperonins. The substrate folding process involves multivalent binding of distinct substrate epitopes by the different TRiC apical domains. Release of substrate may proceed in a sequential fashion owing to the asymmetric usage of ATP by the eukaryotic group II chaperonin and the nonconcerted nature of lid closure. Once encapsulated, substrates experience a polarized charge environment with one lobe of the complex demonstrating a positive electrostatic potential while the other is negative.