Abstract
Purpose
Tongue base retraction during swallowing is critical to bolus propulsion in normal physiological swallowing. A better understanding of the hyoglossus and styloglossus, muscles thought to be key to tongue base retraction, will improve the quality of physical rehabilitation in dysphagic patients in addition to preventing iatrogenic damage to structures critical to deglutition. This study utilized muscle functional MRI in healthy adult human subjects in order to determine if the hyoglossus and styloglossus are active during swallowing.
Methods and Materials
Data were collected for 11 subjects with mfMRI before and after swallowing, and after performing the Mendelsohn maneuver. Whole-muscle relaxation time profiles (T2 signal in milliseconds) were calculated from weighted averages of multiple dual echo MRI slices, allowing for comparison of physiological response for the muscles in each test condition. Changes in effect size (Cohen’s d) of whole-muscle T2 profiles were used to establish whether or not the hyoglossus and styloglossus are utilized during swallowing and during the Mendelsohn maneuver.
Results
Post-swallowing effect size changes (where a d value of >0.20 indicates significant activity) for the T2 signal profiles of the hyoglossus and styloglossus were found to be d = 1.19 and 0.22, respectively. The hyoglossus showed an effect size change of d = 0.26 for the Mendelsohn maneuver.
Conclusions
Muscle functional MRI indicates a physiological response of the hyoglossus and styloglossus during swallowing, and the hyoglossus during the Mendelsohn maneuver.
Keywords: muscle functional MRI, T2-weighted images, styloglossus, hyoglossus, tongue base retraction, swallowing
1. Introduction
Swallowing difficulty poses a threat to health care status and quality of life. An improved understanding of the functional anatomy of swallowing will aid in the physical rehabilitation in dysphagic patients as well as the protection of these structures by surgeons operating in the head and neck or radiation oncologists treating head and neck cancer with Intensity Modulated Radiation Treatment. Deglutition is a complex process is described in three distinct phases including the oral, pharyngeal, and esophageal [1],[2]. At the initiation of the pharyngeal phase, retraction of tongue base provides thrust to the bolus to propel it through the hypopharynx into the esophagus. Reduced tongue base retraction has been associated with incomplete bolus clearance in head and neck cancer patients and is important to airway safety during swallowing [3]. Muscles underlying tongue base retraction are key elements of normal swallowing physiology.
The tongue is a hydrostat composed of intrinsic muscles including the superior and inferior longitudinal muscles, transverse muscle, and vertical muscle and is situated in the oral cavity and pharynx by extrinsic muscles including the genioglossus, hyoglossus, palatoglossus, and styloglossus. The genioglossus attaches the tongue to the mandible, palatoglossus attaches tongue to the palate, the hyoglossus attaches the tongue to the hyoid bone (along with the hyo-glosso-epiglottic ligament), and styloglossus attaches the tongue to the cranial base via the styloid process. Of these muscles, the hyoglossus and styloglossus are thought to be responsible for retracting the tongue base during swallowing (Fig. 1). Electromyography, a technique involving the use of hook wire electrodes to measure muscle activity, has been used to verify the hyoglossus and styloglossus in mammalian models [4],[5]. In humans, previous MRI studies have documented the deformation of the tongue muscles including the hyoglossus during speech via structural or cine-MRI [6],[7]. However, no previous reports have verified the activity of the styloglossus and hyoglossus muscles in human swallowing.
Figure 1.
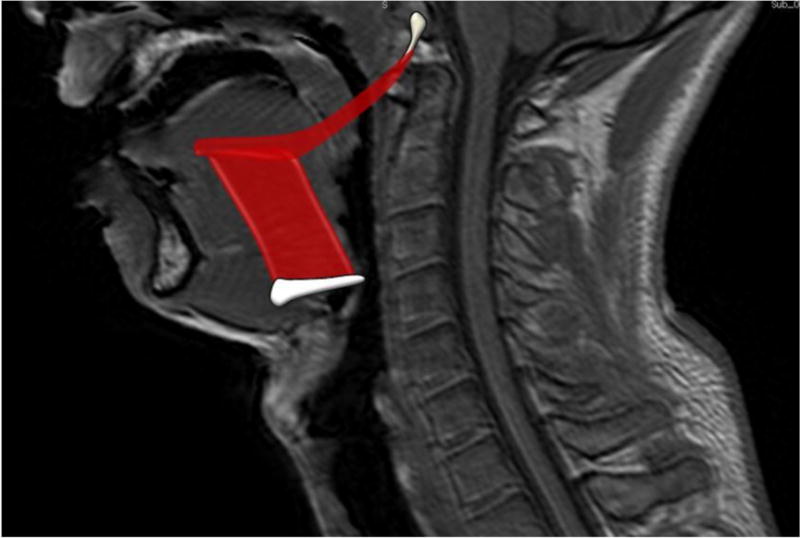
Hyoglossus and Styloglossus Locations and Attachment Sites. The hyoglossus originates from the hyoid bone and attaches to the tongue and styloglossus. The styloglossus originates from the styloid process at the base of the skull and inserts posteriorly to the hyoglossus and tongue.
The aim of this study is to determine if the styloglossus and hyoglossus underlie tongue base retraction associated with deglutition in humans. Our working hypothesis is that these two muscles show an increased physiological response post-swallowing tasks compared to baseline as determined by muscle functional magnetic resonance imaging (mfMRI). The physiological response of the styloglossus and hyoglossus will be determined by comparing T2 signal profiles of muscles: at baseline with post-swallowing; and at baseline with post swallowing exercise (Mendelsohn maneuver). While electromyography is a widely used method for studying local muscle activity [8], mfMRI is less invasive and allows for determination of whole muscle response that are often difficult to access in human subjects.
2. Methods and Materials
Eleven healthy subjects were recruited and consented to the study. Of the 11 subjects, 5 were female and 6 were male, all of them between 22 and 30 years of age (mean age, 25). The Boston University Medical Campus Institutional Review Board originally provided ethics approval for this research protocol; the present study was executed through a data sharing agreement and the approval of the Georgia Regents University Institutional Review Board.
Subjects were trained by the speech language pathologist to perform the Mendelsohn maneuver exercise in the supine position in preparation for positioning required for image acquisition in the MRI scanner. Fiber-optic Endoscopic Examination of Swallowing (FEES) was used to verify normal swallowing physiology and performance of the Mendelsohn maneuver. Prior to scanning, subjects were instructed to perform exercises using visual cues, carried out by projecting PowerPoint presentation slides (Microsoft Corporation, Redmond, WA) into the scanner with mirrors. Repetitive swallowing was self-selected via tubing connected to a reservoir of magnesium infused thin liquid bolus (non-carbonated sports drink).
MR image acquisition was performed without intravenous contrast using a 3 Tesla Achieva MRI scanner (Philips Corporation, Andover, MA) and a 16 channel neurovascular coil. T1-weighted MRI scans were taken in 3 planes for structural cross-referencing with the muscle function imaging. The muscle functional acquisitions were contiguous 4 mm axial scans collected from the cricoid to the hard palate utilizing a spin-echo sequence with a repetition time (TR) of 2500 msec and dual echo times (TE) of 17.8 and 80 msec. Separate image series were taken for each subject before swallowing tasks as a baseline T2 muscle profile measurement, after 8 repeated swallows, and after performing 8 Mendelsohn maneuvers for 5 seconds each. A resting period of 20 minutes between sets of exercises was allotted for each subject. To verify performance of swallowing tasks, a two-planar (coronal and sagittal) dynamic MRI scan (T1-weighted fast gradient echo sequence with TE/TR of 0.9/2.4 msec, 10-mm slice thickness, and temporal resolution of 8.3 fps) was also acquired.
Using semi-automated muscle segmentation for each slice of each image series corresponding to the subject and task, the investigator was able to determine mean signal intensity values for both long and short TE image types. Muscle segmentation was carried out using Osirix digital imaging and communication in medicine software (http://www.osirix-viewer.com/). With each slice of each image series, the investigator first selected a seed point. The confidence algorithm for the growing region of interest compared the signal intensity of the selected voxel, and compared the surrounding 8 voxels in the slice to the center voxel, using the multiplier parameter as the standard deviation and the average signal intensity of the 9 voxels. The multiplier parameter was set at 1.0 standard deviation for larger muscle segments and at 0.5 for smaller muscle segments for selection sensitivity. The plug-in then selected voxels that fell within the specified multiplier distance from the mean, and continued selecting voxels surrounding those initial voxels based on the initial seed point signal intensity average and multiplier (Fig. 2). The result was a portion of tissue of similar signal intensity resembling the anatomy selected. After selections were made, the investigator could accept or reject segmentation selections based on whether or not the selections were maintained within the boundaries of the muscle of interest. In this way, voxels representing muscle tissue versus other tissue types we segmented. The regions of interest for each long TE slice were then copied to the corresponding short TE slice. By recording signal intensities of the long TE and short TE dynamics separately, the investigator remained blinded to the T2 value of the segmentation. The styloglossus and hyoglossus were segmented at every discernable level for each test condition (baseline, post-swallow, and post Mendelsohn maneuver). For consistency, the investigator only segmented muscles on the right side of the subject (Fig. 3).
Figure 2.
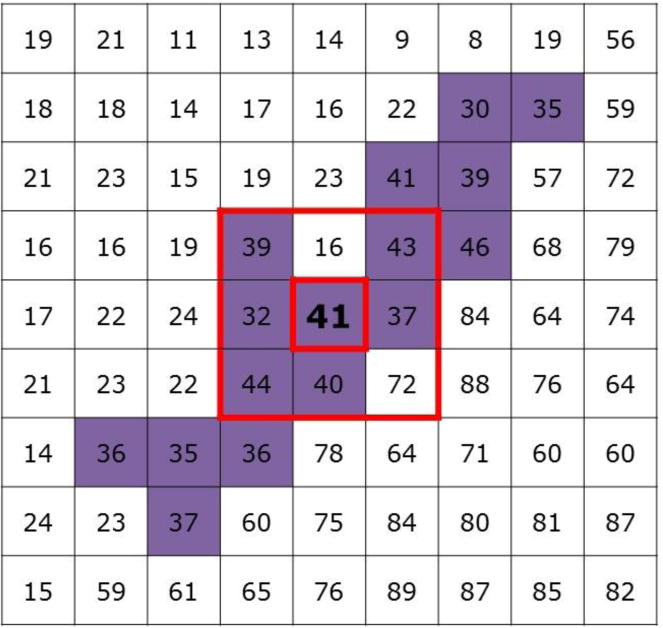
Semiautomated muscle segmentation. The “growing region of interest” interface in Osirix uses the 9 voxels immediately surrounding (outer box) and including a seed point (inner box) selected by the investigator. The algorithm determines the means and standard deviations of signal intensities of these 9 voxels then selects all neighboring voxels falling within a standard deviation of the mean (in purple). The principle is that tissue types with similar signal intensities to the seed point will be segmented while other tissue types are excluded, allowing for the analysis of the muscle tissue.
Figure 3.
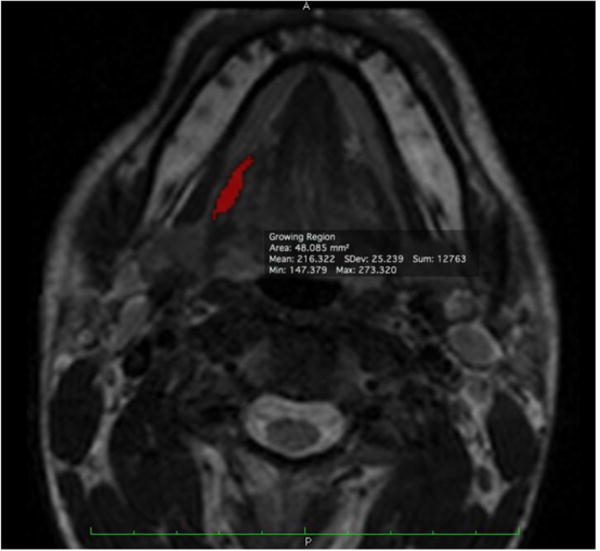
Hyoglossus segmentation example. At one slice level, the completed segmentation of the hyoglossus on the right side of the subject will appear as shown. The voxel selections made by the semiautomated muscle segmentation algorithm in Osirix were accepted or rejected until the final segmentation fit distinct muscle boundaries in the axial T2-weighted MR image series.
Using T1 weighted images in three planes to verify the hyoglossus and styloglossus, a protocol for muscle segmentation was developed as follows. To segment the hyoglossus, the hyoid bone was first identified. Advancing one or two slices superiorly (4–8 mm), the submandibular gland, mylohyoid, submandibular duct, posterior belly of the digastric, and hyoglossus could be distinctly identified. Muscle segmentation began at that level, continuing superiorly as the muscle body locates anterosuperiorly to its attachment sites on the tongue and styloglossus. Segmentation for the hyoglossus specifically ended one slice before the styloglossus was visualized extending posteriorly to the tongue. Styloglossus muscle segmentation began at the most clearly distinguishable inferior attachment site, which was at or 1 slice inferior to where hyoglossus muscle segmentation ended, with the styloglossus attaching to the hyoglossus posteriorly. Segmentation then continued superiorly, with the largest segmentation area being the muscle fibers sweeping posteriorly to the tongue. As the styloglossus was followed more superiorly, the muscle belly became smaller, tracking around the medial pterygoid muscle posterolaterally until reaching its common attachment point with other styloid muscles. The styloglossus was not segmented at the styloid process to avoid inclusion of other muscles.
To test intra-rater reliability, 25% of subjects were re-segmented and signal intensities and segmentation centroid coordinates were compared. Pearson correlations indicated a reliability of r=.998 and r=.996 for signal intensities for hyoglossus and styloglossus respectively. Centroid location results were r=.93 r=.98, for x and y hyoglossus coordinates respectively, and r=.91, r=.86 for x and y styloglossus coordinates respectively.
To control for size variation of muscles from slice to slice, a T2 whole muscle profile was calculated by weighting the mean signal intensity of each slice with the number of voxels selected against the total number of voxels in the muscle across multiple slices. The whole muscle T2 profile for each muscle was then calculated using the following formula for each test condition:
where T is the calculated T2 value, TElong as 80 msec, TEshort as 17.8 msec, SIshort as the mean weighted signal intensity of the TEshort images, and SIlong as the mean weighted signal intensity of the TElong images.
Negative and positive control data of the physiological response using whole muscle T2 profiles from this data set was reported in a previous study [9]. The negative control compared sternocleidomastoid muscles at baseline and post-swallowing and found no significant changes. A positive control was also incorporated as a bite down task including 2 subjects that showed significant increases in the response of the masseter muscle.
Comparisons of muscle activation between baseline and post-swallowing task conditions were made using a repeated measures Cohen’s d to evaluate effect size changes [10]. Cohen’s d value significance was set at greater than 0.20, and post hoc corrections to effect sizes were made for a sample size of less than 20.
3. Results
Whole muscle T2 signal profile means and standard deviations for the hyoglossus and styloglossus under each condition (baseline, post-swallowing and post Mendelsohn maneuver) are reported in Table 1. Effect size changes in whole muscle T2 signal profile for hyoglossus was d=1.19 for baseline vs. post-swallowing groups and d=0.26 in baseline vs. post Mendelsohn maneuver groups. Effect size changes in whole muscle T2 signal profile for styloglossus was d=0.22 for baseline vs. post-swallowing groups and d=0.06 in baseline vs. post Mendelsohn maneuver groups.
Table 1.
Whole Muscle T2 Signal Profile Means and Standard Deviations by Task
| Muscle | Task | Mean (msec) | Standard Deviation |
|---|---|---|---|
| Hyoglossus | Baseline | 48.49 | 2.11 |
| Post-Swallowing | 50.37 | 3.16 | |
| Post-Mendelsohn | 49.03 | 4.39 | |
|
| |||
| Styloglossus | Baseline | 47.46 | 1.83 |
| Post-Swallowing | 47.95 | 3.19 | |
| Post-Mendelsohn | 47.59 | 3.02 | |
4. Discussion
Effect size changes in this sample indicate that styloglossus and hyoglossus are active during swallowing in humans. These findings are consistent with what has been found in animal models using electromyography [11],[4]. MRI has been used in humans to measure directional strain in the tongue implicating the styloglossus and hyoglossus in swallowing [12]. The present study supports the findings of these prior studies.
The styloglossus showed a small effect size change following the swallowing task as compared to the baseline. Small effect size changes in the styloglossus likely indicate a small physiological response that correlates with efficient muscle use in normal physiology as documented in other swallowing muscles [9]. It is plausible that the physical constraint of subjects swallowing in the supine position allows for a bolus to fall into the hypopharynx without strong recruitment of the styloglossus. Kitamura and colleagues found that when comparing tongue position in upright vs conventional MRI scanners, the tongue was retracted by gravitational forces in the supine position [13].
A large effect size change was found in the hyoglossus muscle with the comparison of baseline and post-swallowing tasks. Such a difference in effect size compared to the apparent efficiency of the styloglossus suggests a larger effort of the hyoglossus. It is likely that this larger response correlates with the experimental set up wherein a subject self-selects bolus sizes through a tubing apparatus requiring some suction. An electromyography study in pigs found heightened hyoglossus activity during suck-swallow cycles [4]. The combined suction and swallowing activities may have then increased the whole muscle T2 profile of the hyoglossus, in turn causing the effect size change to be much larger in the post-swallow task compared to styloglossus.
Significant differences were not seen between the baseline and Mendelsohn maneuver for the styloglossus though small effect size changes were found for the hyoglossus. Lazarus and colleagues found that several exercises including the supersupraglottic swallow, effortful swallow, Mendelsohn maneuver, and tongue-hold maneuver improved tongue base retraction function [14]. In a previous study the Mendelsohn maneuver was shown to recruit muscles underlying hyolaryngeal elevation excepting the geniohyoid [9]. This evidence seems to suggest that the styloglossus may only be recruited for swallowing tasks that include tongue base retraction whereas the hyoglossus is likely to be multifunctional. It may be that the tongue hold maneuver would specifically target the styloglossus [15]. Muscle functional MRI would be a useful method to determine what which muscles or muscle groups are targeted by rehabilitative exercises.
5. Conclusions
This study utilized muscle functional magnetic resonance imaging to calculate whole muscle T2 profiles to compare baseline, post-swallowing, and post Mendelsohn maneuver tasks to determine the physiological response of the hyoglossus and styloglossus to swallowing tasks in humans. This methodology allows for the analysis of muscles not easily accessible in humans by hook wire electrodes necessitated by electromyography. The hyoglossus and styloglossus muscles were shown to be active during swallowing and the hyoglossus during a swallowing exercise known as the Mendelsohn maneuver.
Supplementary Material
Acknowledgments
The authors acknowledge the expertise of Ron Killiany, Ph.D., the staff of the Boston University School of Medicine Center for Biomedical Imaging for their assistance in this study. The project was made possible by funding from the Medical Scholars Program at the Medical College of Georgia at Georgia Regents University. Data collection for this study was supported in part by National Institutes of Health (NIH)/National Institute on Deafness and Other Communication Disorders (NIDCD) grant F31DC011705 (to W.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clinical Neurophysiology. 2003;114(12):2226–2244. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Physical medicine and rehabilitation clinics of North America. 2008;19(4):691–707. vii. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pauloski BR, Logemann JA. Impact of tongue base and posterior pharyngeal wall biomechanics on pharyngeal clearance in irradiated postsurgical oral and oropharyngeal cancer patients. Head & neck. 2000;22(2):120–131. doi: 10.1002/(sici)1097-0347(200003)22:2<120::aid-hed3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Thexton AJ, Crompton AW, German RZ. EMG activity in hyoid muscles during pig suckling. Journal of applied physiology. 2012;112(9):1512–1519. doi: 10.1152/japplphysiol.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutlive TG, McClung JR, Goldberg SJ. Whole-muscle and motor-unit contractile properties of the styloglossus muscle in rat. Journal of neurophysiology. 1999;82(2):584–592. doi: 10.1152/jn.1999.82.2.584. [DOI] [PubMed] [Google Scholar]
- 6.Takano S, Honda K. An MRI analysis of the extrinsic tongue muscles during vowel production. Speech communication. 2007;49(1):49–58. [Google Scholar]
- 7.Stone M, Davis EP, Douglas AS, et al. Modeling the motion of the internal tongue from tagged cine-MRI images. The Journal of the Acoustical Society of America. 2001;109(6):2974–2982. doi: 10.1121/1.1344163. [DOI] [PubMed] [Google Scholar]
- 8.Doble EA, Leiter JC, Knuth SL, Daubenspeck J, Bartlett D. A noninvasive intraoral electromyographic electrode for genioglossus muscle. Journal of applied physiology. 1985;58(4):1378–1382. doi: 10.1152/jappl.1985.58.4.1378. [DOI] [PubMed] [Google Scholar]
- 9.Pearson WG, Jr, Hindson DF, Langmore SE, Zumwalt AC. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. International journal of radiation oncology, biology, physics. 2013;85(3):735–740. doi: 10.1016/j.ijrobp.2012.07.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological methods. 2002;7(1):105. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 11.Tomomune N, Takata M. Excitatory and inhibitory postsynaptic potentials in cat hypoglossal motoneurons during swallowing. Experimental brain research. 1988;71(2):262–272. doi: 10.1007/BF00247486. [DOI] [PubMed] [Google Scholar]
- 12.Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Biomechanical basis for lingual muscular deformation during swallowing. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1999;277(3):G695–G701. doi: 10.1152/ajpgi.1999.277.3.G695. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura T, Takemoto H, Honda K, et al. Difference in vocal tract shape between upright and supine postures: Observations by an open-type MRI scanner. Acoustical Science and Technology. 2005;26(5):465–468. [Google Scholar]
- 14.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatrica et Logopaedica. 2002;54(4):171–176. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 15.Fujiu M, Logemann JA. Effect of a tongue-holding maneuver on posterior pharyngeal wall movement during deglutition. American Journal of Speech-Language Pathology. 1996;5(1):23–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


