Abstract
Background:
Mycoplasma hominis and Ureaplasma urealyticum are important causative agents of vaginitis, cervicitis, postpartum sepsis, reproductive infections and infertility in both males and females.
Objectives:
According to the uncertain prevalence of U. urealyticum and M. hominis in Iranian infertile females, the present study was carried out to determine the prevalence of U. urealyticum and M. hominis in high vaginal swab samples of fertile and infertile females.
Patients and Methods:
A total of 350 high vaginal swab specimens were taken from fertile and infertile females. Samples were cultured and those that were positive for bacteria were subjected to the polymerase chain reaction (PCR) for further confirmation.
Results:
Of the 350 collected samples, eleven were positive for M. hominis (3.14%), fifteen were positive for U. urealyticum (4.28%) and five were positive for both of them (1.42%). Prevalence of U. urealyticum and M. hominis in the high vaginal parts of infertile females was higher than fertile females (P < 0.05). The results of traditional method were also confirmed, using the PCR amplification of urease gene of U. urealyticum and 16SrRNA gene of the M. hominis. Ureaplasma urealyticum and M. hominis had a higher prevalence in the high vaginal samples collected during the summer season.
Conclusions:
Considerable prevalence of M. hominis and U. urealyticum in the high vaginal swab samples of infertile females compared to the low prevalence in fertile females may suggest that these two pathogens can be cause infertility. Application of the PCR method is recommended for rapid and sensitive detection of M. hominis and U. urealyticum in high vaginal swab samples.
Keywords: Prevalence, Infertility, Female, Iran, Mycoplasma hominis, Ureaplasma urealyticum
1. Background
One of the main issues dealt by gynecologists is infertility. Infertility is considered when couples have been trying to become pregnant with frequent intercourse for at least a year with no success (1-4). Documented data revealed that approximately 72.4 million couples are infertile (4). The main reasons for 25% of infertility cases are still unknown (1-4). The majority of females with infertility problems are afflicted with inflammatory changes of the oviduct or surrounding peritoneum. Most of these alterations result from infections (5).
Mycoplasma hominis and Ureaplasma urealyticum have been isolated from genital mucosal surfaces, vagina and cervical parts of females (6, 7). They have also been isolated from cases of genital infections including both males and females (8). Ureaplasma urealyticum is a major cause of non-chlamydial and non-gonococcal urethritis, chorioamnionitis, acute prostatitis, vaginitis, cervicitis, preterm delivery and sepsis (8, 9). Mycoplasma hominis is often associated with vaginitis, cervicitis, postpartum sepsis, pyelonephritis, preterm labor and premature birth (10, 11).
Bacterial vaginosis is strongly implicated in female infertility and it probably is an underestimated cause of unexplained infertility. It was also documented that screening and treatment of bacterial vaginosis during the course of infertility treatment increased the rate of pregnancy (12). Therefore, it is important to know the exact prevalence rate of infectious agents that cause vaginitis in infertile women. In keeping with this, safe, rapid, accurate and sensitive diagnosis of these infectious agents can help medical practitioners reduce the risk of vaginitis and infertility.
2. Objectives
The exact prevalence and epidemiology of M. hominis and U. urealyticum is still unknown in Iran. Therefore, the present investigation was carried out in order to determine the prevalence rate of M. hominis and U. urealyticum in high vaginal swab samples of infertile females, who had been referred to Iranian sterility and infertility hospitals due to their infertility problems.
3. Patients and Methods
3.1. Ethical Issues
The present study was approved by the ethical committee of various infertility and sterility centers of Iran. The authors made every attempt to protect the life, health, dignity, integrity, and rights to self-determination, privacy and confidentiality of the personal information of the studied females. We conformed to generally accepted scientific principles, based on a thorough knowledge of the scientific literature, other relevant sources of information, and adequate laboratories. All samples were taken from volunteers who were referred to various infertility and sterility hospitals of Iran.
3.2. Samples Collection
From October 2013 to October 2014, a total of 150 high vaginal swab specimens were taken from infertile females. Generally, a female who was not pregnant after a year of regular intercourse without prevention was defined as infertile. Vaginal specimens were collected from the ventral fornix without any contact with urine and external parts of the reproductive system using speculum and commercial sterile cotton-tipped swabs. All specimens were collected by an expert midwife. Two-hundred vaginal swab specimens were also collected from fertile females with no history of infertility.
3.3. Ureaplasma urealyticum and Mycoplasma hominis identification
In order to identify the bacteria, the method described by Bayraktar et al. (2010) (13) was used. Mycoplasma hominis and U. urealyticum were detected using a commercial kit, Mycoplasma IST-2 (BioMerieux, Marcy l’Etoile, France), according to the manufacturer’s instructions. The kit contained strips that gave information about the presence or absence of M. hominis and U. urealyticum. One strip was placed directly into R1 tubes (transport medium) and subsequently delivered to the clinical laboratory for the identification of both U. urealyticum and M. hominis.
Swabs in the R1 transport medium were processed according to the manufacturer’s instructions. They were vortexed rapidly, and 3 mL of R1 was used to rehydrate the lyophilized growth medium R2 (provided in the Mycoplasma IST-2 kit). A mycoplasma IST strip, consisting of 22 wells, was then inoculated with the rehydrated R2 growth medium (55 mL per well, overlaid with two drops of mineral oil). From the R2 positive tube, 0.1 mL was also inoculated onto A7 Mycoplasma agar plates (BioMerieux, Marcy l’Etoile, France) and incubated at 37°C in an atmosphere of 5% CO2 for checking characteristic colony morphology. All media and the inoculated strip were incubated at 37°C in a CO2 incubator and observed for color changes, and the results were interpreted after 24 and 48 hours of incubation. Wells provided information on the presence or absence of M. hominis and U. urealyticum, with an estimate of the density of each organism (≥ 104 CFU). The A7 plates were examined with a microscope twice daily for up to five days for characteristic colonies. Colonies presenting a fried egg appearance suggested the presence of M. hominis, whereas colonies that were brown and tiny indicated the presence of U. urealyticum. Mycoplasma hominis ATCC 23114 and U. urealyticum ATCC 27618 strains were used as controls.
3.4. Polymerase Chain Reaction Confirmation of Ureaplasma urealyticum and Mycoplasma hominis
A PCR technique was used in order to detect U. urealyticum and M. hominis (14, 15). Total genomic DNA was extracted from the bacterial colonies using the commercial genomic DNA extraction kit (Fermentas, Germany), according to the manufacturer’s instructions. The DNA concentration was determined by measuring absorbance of the sample at 260 nm using a spectrophotometer (16). Table 1 shows the oligonucleotide primers, size of products and PCR conditions used for detection of U. urealyticum and M. hominis. The PCR amplification products (15 μL) were subjected to electrophoresis on 1.5% agarose gel in 1X Tris/Borate/EDTA (TBE) buffer at 80 V for 30 minutes, stained with SYBR Green. All runs included a negative DNA control consisting of PCR grade water, and strains of M. hominis ATCC 23114 and U. urealyticum ATCC 27618 were used as positive controls.
Table 1. The Oligonucleotide Primers and the Polymerase Chain Reaction Programs Used for Amplification of Urease Gene of the Ureaplasma urealyticum and 16SrRNA gene of the Mycoplasma hominis (14,15).
| Target Gene | Primer Sequence (5’ - 3’)* | PCR Product, bp | PCR Programs | PCR Volume, 50µL |
|---|---|---|---|---|
| Urease gene of the U. urealyticum | 429 | 1 cycle: 95 °C at 3 minutes; 30 cycle: 95 °C at 20 seconds; 58 °C at 40 seconds; 72 °C at 30 seconds. | 5 µL PCR buffer 10×;1.5 mM Mgcl2; 200 µM dNTP (Fermentas); 0.5 µM of each primers F & R; 1.25 U Taq DNA polymerase (Fermentas); 2.5 µL DNA template. | |
| F | ACGACGT CCATAAGCAACT | |||
| R | CAATCTGCTCGTGAAGTATTAC | |||
| 16SrRNA gene of the M. hominis | 344 | 1 cycle: 95 °C at 3 minutes; 30 cycle: 95 °C at 20 seconds; 58 °C at 40 seconds; 72 °C at 30 seconds; 1 cycle: 72 °C at 8 minutes | 5 µL PCR buffer 10×;1.5 mM Mgcl2; 200 µM dNTP (Fermentas); 0.5 µM of each primers F & R; 1.25 U Taq DNA polymerase (Fermentas); 2.5 µL DNA template | |
| F | CAA TGG CTA ATG CCG GAT ACG C | |||
| R | GGT ACC GTC AGT CTG CAA T |
3.5. Statistical Analysis
Statistical analysis was performed using the SPSS version 16.0 software for significant relationships. The incidences of M. hominis and U. urealyticum in fertile and infertile cases were statistically analyzed. Statistical significance was regarded at a P value of < 0.05.
4. Results
A total of 150 high vaginal swab samples taken from the infertile females and also 200 high vaginal swab samples of fertile females were analyzed for the presence of M. hominis and U. urealyticum (Table 1). Of the 350 collected samples, 11 samples were positive for M. hominis (3.14%) and 15 samples were positive for U. urealyticum (4.28%). A statistically significant difference was seen between the prevalence of M. hominis and U. urealyticum (P < 0.05). A significant difference was also seen between the type of samples and prevalence of bacteria (P < 0.05). Overall, five out of 350 samples (1.42%) were infected with both M. hominis and U. urealyticum. Samples taken from the high vaginal parts of the infertile females had a higher prevalence of M. hominis and U. urealyticum than those taken from fertile females (P < 0.05). The results of the conventional technique were confirmed using the PCR method.
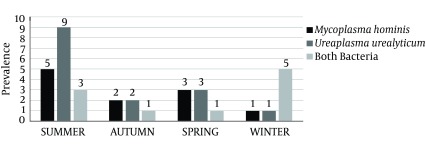
Seasonal distribution of M. hominis and U. urealyticum is shown in Figure 1. Results showed that M. hominis and U. urealyticum had a higher prevalence in the high vaginal samples collected during the summer season, followed by spring and autumn. A statistically significant difference was seen in the prevalence of bacteria between hot and cold seasons of the year (P < 0.05).
Figure 1. Seasonal Distribution of Mycoplasma hominis and Ureaplasma urealyticum in Fertile and Infertile Females.
Table 2. Prevalence of Mycoplasma hominis and Ureaplasma urealyticum in the High Vaginal Swab Samples of Fertile and Infertile Females.
| Types of High Vaginal Swab Samples | No Samples Collected | No. Bacteria (%) | ||
|---|---|---|---|---|
| No. M. hominis | No. U. urealyticum | Both Bacteria | ||
| Fertile females | 200 | 3 (1.5) | 5 (2.5) | 1 (0.5) |
| Infertile females | 150 | 8 (5.33) | 10 (6.66) | 4 (2.66) |
| Total | 350 | 11 (3.14) | 15 (4.28) | 5 (1.42) |
5. Discussion
The results of our study showed that M. hominis and U. urealyticum had a considerable prevalence in the high vaginal swab samples collected from the infertile females referred to Iranian hospitals and health centers. Our results showed that the prevalence of M. hominis and U. urealyticum in fertile and infertile females were 3.14% and 4.28%, respectively. The prevalence of both bacteria was higher in samples taken from infertile females than those of fertile controls. One possible explanation for the higher prevalence of bacteria in the infertile females is hormonal disorders. In fact, hormonal disorders that occur in infertile females can lead to reduced levels of immunity and increase bacterial colonization and survival in vaginal epithelium (17). A previous study showed that the levels of Igs G, A, M and SIgA, IgA, and lysozyme were decreased in infertile females (18). Pelzer et al. (2011) reported that female follicular fluid is not sterile and the levels of cytokines, chemokines and growth factors, which are essential for ovarian function (18), secretion of ovarian steroid hormones (19) and development and regression of corpus luteum (20), were decreased in follicular fluid of infertile females.
In addition to the considerable prevalence of M. hominis and U. urealyticum in high vaginal swab samples of our study, several previously published reports revealed various prevalence rates for these bacteria. Akya et al. (2014) (21) reported that the prevalence of M. hominis and U. urealyticum were 34.9% and 6%, respectively, which was higher than our results. The prevalence rate of M. hominis and U. urealyticum in vaginal swabs ranged from zero to 3.8% (22, 23). The prevalence of U. urealyticum was reported from 20% in South America to 41.9% and 51.5% in Italy and Africa, respectively (22). Zdrodowska-Stefanow et al. (2006) (24) and Rodriguez et al. (2001) (25) reported higher prevalence rates of U. urealyticum infection (37-50%). In keeping with this, the prevalence of these bacteria was lower in some other studies (23, 26). Peerayeh et al. (2007) (27) reported that of the 377 patients studied, 141 samples (37.4%) were positive for U. urealyticum, which was higher than our results. A previous study from Ahvaz, Iran (28) showed that the prevalence of M. hominis and U. urealyticum in the genital samples of females were 6.4% and 13.6%, respectively. They showed that the highest prevalence of M. hominis (54.5%) and U. urealyticum (53.8%) was found in females within 28 - 33 and 34 - 39 years old. Another study from Iran (29) showed that the prevalence of M. hominis from vaginal and cervical parts of 104 patients aged 18 to 48 years were 30% and 21%, respectively, both being higher than our findings.
The different prevalence rates reported by previous studies may be due to factors, including the population tested (whether the subjects had received antibiotics, had a genital infection or not, and the number of sexual partners, etc.), type of samples (vaginal swabs, cervical swabs, urine etc.), number of samples collected, methods of sampling, experimental methods, age, socioeconomic status, geographical area, season of sampling and even climate of the sampled area.
Our findings also revealed that the majority of bacterial strains were recovered from high vaginal samples collected during the summer season. The main reason for the highest prevalence of M. hominis and U. urealyticum in summer is the fact that during this time climatic events, heat, rain, and thunderstorms, as well as variation of barometric pressure may have an influence on the prevalence of bacteria. Bacteria have higher growth rate and surveillance under warm conditions. After analyzing the average temperatures of the region of sampling (16°C for spring, 31°C for summer, 13°C for autumn and 4°C for winter), it was determined that the prevalence rate of M. hominis and U. urealyticum strains during each season is related to the average temperatures. This study is the first prevalence report of seasonal distribution of M. hominis and U. urealyticum in high vaginal swab samples of infertile females.
The present study showed the considerable prevalence of M. hominis and U. urealyticum in high vaginal swab samples of infertile and fertile females from Iran. Considering the high prevalence rate (3.14% for M. hominis and 4.28% for U. urealyticum), there is a need to use a rapid diagnostic method. The PCR technique using the urease gene of the U. urealyticum and 16SrRNA gene of the M. hominis, which was applied in this study, could be used as a safe, sensitive, specific and fast method for diagnosis of M. hominis and U. urealyticum. The authors of the present study recommended precise monitoring and control of fertile and infertile females for the presence of M. hominis and U. urealyticum and treatment of positive cases to prevent infection and possibly infertility.
Footnotes
Authors’ Contribution:Mehri Seifoleslami developed the original idea and designed the protocol. Aghdas Safari analyzed and interpreted the data. Maryam Khayyat Khameneie supervised the study and prepared and revised the manuscript.
References
- 1.Jensen JS. Mycoplasma genitalium infections. Diagnosis, clinical aspects, and pathogenesis. Dan Med Bull. 2006;53(1):1–27. [PubMed] [Google Scholar]
- 2.Khalili MA, Pourshafiei MR, Saifi M, Khalili MB. Bacterial infection of the reproductive tract of infertile men in Iran. Mid East Fertil Soc J. 2000;51:26–31. [Google Scholar]
- 3.Daar AS, Merali Z. Infertility and social suffering: the case of ART in developing countries. In: Vayena E, Rowe P, Griffin D, editors. Report of a meeting on "medical, ethical, and social aspects of assisted reproduction. Geneva: WHO; 2001. pp. 16–21. [Google Scholar]
- 4.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–12. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 5.Wiesenfeld HC, Hillier SL, Meyn LA, Amortegui AJ, Sweet RL. Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol. 2012;120(1):37–43. doi: 10.1097/AOG.0b013e31825a6bc9. [DOI] [PubMed] [Google Scholar]
- 6.Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis. 2013;26(3):231–40. doi: 10.1097/QCO.0b013e328360db58. [DOI] [PubMed] [Google Scholar]
- 7.Wang QY, Li RH, Zheng LQ, Shang XH. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in female outpatients, 2009–2013. J Microbiol, Immunol Infection. 2009-2013. doi: 10.1016/j.jmii.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Al-Sweih NA, Al-Fadli AH, Omu AE, Rotimi VO. Prevalence of Chlamydia trachomatis, Mycoplasma hominis, Mycoplasma genitalium, and Ureaplasma urealyticum infections and seminal quality in infertile and fertile men in Kuwait. J Androl. 2012;33(6):1323–9. doi: 10.2164/jandrol.111.013821. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, Liu J, Ling Y, Dong C, Wu T, Yu X, et al. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Chinese women with genital infectious diseases. Indian J Dermatol, Venereology, Leprology. 2012;78(3):406–7. doi: 10.4103/0378-6323.95480. [DOI] [PubMed] [Google Scholar]
- 10.Aydin Y, Atis A, Ocer F, Isenkul R. Association of cervical infection of Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis with peritoneum colonisation in pregnancy. J Obstet Gynaecol. 2010;30(8):809–12. doi: 10.3109/01443615.2010.519063. [DOI] [PubMed] [Google Scholar]
- 11.Campos GB, Lobao TN, Selis NN, Amorim AT, Martins HB, Barbosa MS, et al. Prevalence of mycoplasma genitalium and mycoplasma hominis in urogenital tract of brazilian women. BMC Infect Dis. 2015;15(1):60. doi: 10.1186/s12879-015-0792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salah RM, Allam AM, Magdy AM, Mohamed A. Bacterial vaginosis and infertility: cause or association? Eur J Obstet Gynecol Reprod Biol. 2013;167(1):59–63. doi: 10.1016/j.ejogrb.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Bayraktar MR, Ozerol IH, Gucluer N, Celik O. Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. Int J Infect Dis. 2010;14(2):e90–5. doi: 10.1016/j.ijid.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17 Suppl 1:S148–53. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard A, Yanez A, Dybvig K, Watson HL, Griffiths G, Cassell GH. Evaluation of intraspecies genetic variation within the 16S rRNA gene of Mycoplasma hominis and detection by polymerase chain reaction. J Clin Microbiol. 1993;31(5):1358–61. doi: 10.1128/jcm.31.5.1358-1361.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrok JA. Molecular cloning, a laboratory manual. 3rd ed. New York, USA: Cold Spring Harbor Laboratory Press; 2001. p. 2100. [Google Scholar]
- 17.Kornats'ka AH. [Local humoral immunity in women with combined forms of infertility]. Lik Sprava. 1998;(4):82–4. [PubMed] [Google Scholar]
- 18.Pelzer ES, Allan JA, Cunningham K, Mengersen K, Allan JM, Launchbury T, et al. Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Hum Reprod. 2011;26(7):1799–812. doi: 10.1093/humrep/der108. [DOI] [PubMed] [Google Scholar]
- 19.Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol. 2002;16(3):580–99. doi: 10.1210/mend.16.3.0806. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Peng Z. [Study of estrogen and progesterone receptors in endometrial carcinoma]. Hua Xi Yi Ke Da Xue Xue Bao. 2000;31(1):98–100. [PubMed] [Google Scholar]
- 21.Akya A, Aletaha M, Ghadiri K, Rezaee M. The frequency of mycoplasma hominis, mycoplasma genitalium and urea plasma urealyticum in women with cervicitis. Journal NI. 2014;1(2):31–7. [Google Scholar]
- 22.Leli C, Mencacci A, Bombaci JC, D'Alo F, Farinelli S, Vitali M, et al. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in a population of Italian and immigrant outpatients. Infez Med. 2012;20(2):82–7. [PubMed] [Google Scholar]
- 23.Elias M, Grzesko J, Siejkowski R, Nowicka J, Maczynska B, Goluda M, et al. [The presence of Mycoplasma hominis and Ureaplasma urealyticum in the cervical canal of uterus]. Ginekol Pol. 2005;76(1):28–32. [PubMed] [Google Scholar]
- 24.Zdrodowska-Stefanow B, Klosowska WM, Ostaszewska-Puchalska I, Bulhak-Koziol V, Kotowicz B. Ureaplasma urealyticum and Mycoplasma hominis infection in women with urogenital diseases. Adv Med Sci. 2006;51:250–3. [PubMed] [Google Scholar]
- 25.Rodriguez R, Hernandez R, Fuster F, Torres A, Prieto P, Alberto J. [Genital infection and infertility]. Enferm Infecc Microbiol Clin. 2001;19(6):261–6. doi: 10.1016/s0213-005x(01)72632-8. [DOI] [PubMed] [Google Scholar]
- 26.Clausen HF, Fedder J, Drasbek M, Nielsen PK, Toft B, Ingerslev HJ, et al. Serological investigation of Mycoplasma genitalium in infertile women. Hum Reprod. 2001;16(9):1866–74. doi: 10.1093/humrep/16.9.1866. [DOI] [PubMed] [Google Scholar]
- 27.Peerayeh SN, Samimi R. Detection of ureaplasma urealyticum in clinical samples from infertile women by polymerase chain reaction. Iranian J Pharmacol Therapeut. 2007;6(1):23–6. [Google Scholar]
- 28.Maleki S, Motamedi H, Moosavian SM, Shahbaziyan N. Frequency of mycoplasma hominis and ureaplasma urealyticum in females with urogenital infections and habitual abortion history in ahvaz, iran; using multiplex PCR. Jundishapur J Microbiol. 2013;6(6) [Google Scholar]
- 29.Ghorbanalinezhad E, Amirmozafari N, Akhavan Sepahi A, Khavari-nejad R. Survey on the genital Mycoplasmosis by multiplex PCR. Int J Mol Clin Microbiol. 2014;(2):451–6. [Google Scholar]