Figure 12.
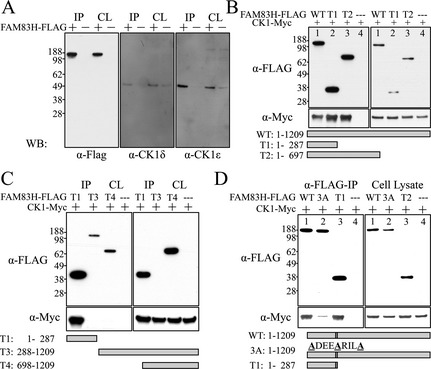
FAM83H‐CK1 interactions. A: Mouse FAM83H‐FLAG was expressed in HEK293 cells and immuno‐precipitated (IP) from cell lysates with α‐FLAG antibodies. The IP products and initial cell lysates (CL) were characterized by western blot analyses using α‐FLAG (left), α‐CK1δ (middle), and α‐CK1ε (right) antibodies. Bands at ~140‐kDa (FAM83H), ~49‐kDa (CK1δ), and ~47‐kDa (CK1ε) were observed. FLAG‐tagged FAM83H (+), but not controls (−), precipitated endogenous CK1δ and CK1ε, suggesting FAM83H interacts with these forms of CK1. B: CK1‐Myc (~50‐kDa) and one of three FAM83H‐FLAG proteins (WT: aa 1–1209, ~140‐kDa; T1: aa 1–287, ~36‐kDa; T2: aa 1–697, ~75‐kDa) or FLAG‐tag only (−) were expressed in HEK293 cells and α‐FLAG immunoprecipitated. The IP products (left) and the initial cell lysates (CL) were immunoblotted with α‐FLAG (top) or α‐Myc (bottom) antibodies. All three different‐length FAM83H‐FLAG proteins pulled down CK1‐Myc, suggesting that the first 287 amino acids of FAM83H are sufficient for the FAM83H‐CK1 interaction. C: Pull‐down assays were performed with three different truncated FAM83H‐FLAG proteins: T1: aa 1–287, ~36‐kDa; T3: aa 288–1209, ~110‐kDa; and T4: aa 698–1209, ~65‐kDa. While T1 can pull down CK1‐Myc, neither N‐terminal truncation (T3 or T4) can, confirming that the first 287 amino acids of FAM83H are necessary for FAM83H‐CK1 interactions. D: Pull down using FAM83H‐FLAG (WT), mutated FAM83H‐FLAG (3A) in which Phe270, Phe274, and Phe278 are all substituted with Ala (WT: F270‐X‐X‐X‐F274‐X‐X‐X‐F278 to 3A: A270‐X‐X‐X‐A274‐X‐X‐X‐A278). Compared to wild‐type (WT) FAM83H, FAM83H3F has significantly reduced ability to pull down CK1, suggesting the F270‐X‐X‐X‐F274‐X‐X‐X‐F278 motif is critical for FAM83H‐CK1 interaction. This motif localizes in the red α‐helix of the human FAM83H1‐287 dimerization model shown on Fig. 11C.
