Figure 7.
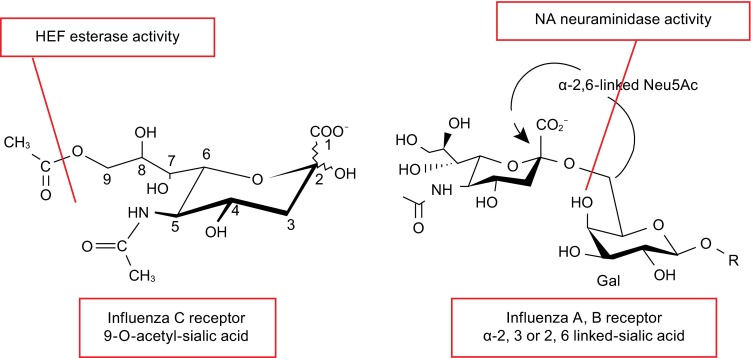
Cellular receptors and receptor-destroying activity of influenza C virus and influenza A and B virus. The structure of cellular receptors for HEF from influenza C virus (N-acetyl-9-O-acetylneuraminic acid) and HA from influenza A and B virus (N-acetylneuraminic acid) are shown. Both neuraminic acid derivatives are the terminal sugars in carbohydrate chains attached to glycolipids or glycoproteins located at the cellular surface. Subtypes of influenza A virus HA discriminate between an α2-6 and α2-3 linkage to the second galactosyl residue, a property that (partially) explains species specificity. HEF of influenza C virus apparently recognizes N-acetyl-9-O-acetylneuraminic acid independent of its linkage to the next sugar. HEF has also esterase activity that cleaves acetyl from the C9 position. In influenza A and B virus the receptor-destroying activity is performed by the NA protein, which hydrolyzes the glycosidic bond between sialic acid and galactosyl residues. The cleaved bonds are indicated by a red line
