Figure 9.
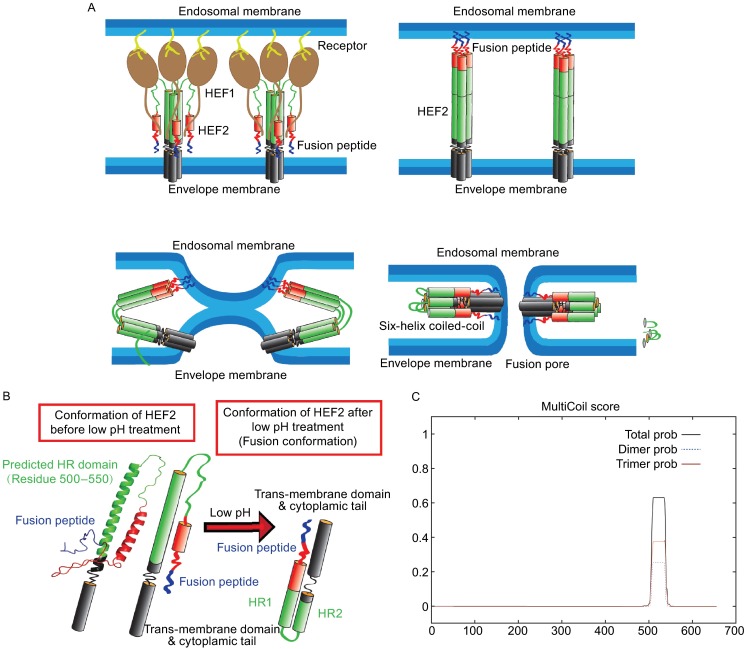
Probable mechanism for HEF-mediated membrane fusion. (A) Probable conformational change of HEF2 during membrane fusion. Left part: structure of the HEF2 subunit at neutral pH. The HR is located in the long α-helix of HEF2 and thus in a similar position as the HR in HA. Right part: hypothetical structure of the HEF2 subunit at acidic pH. (B) Hypothetical scheme for the HEF-catalyzed membrane fusion mechanism. Upper left part: HEF binds to its receptor via its HEF1 subunit (brown) and is endocytosed. Upper right part: Acidification of the endosome causes a conformational shift in HEF2. The fusion peptide, which was (partially) buried in the stalk is exposed and inserts into the endosomal membrane. Lower, left part: The middle part of the HR domain (green) changes its conformation from a helix to a loop, which causes bending of the molecule and close apposition of viral and cellular membrane allowing the exchange of lipids (hemifusion). Lower, right part: Interactions between the fusion peptide and the TMD of HEF might cause opening of a fusion pore. (C) Prediction of a heptad repeat (HR) in HEF by online software “multicoils scoring form”
